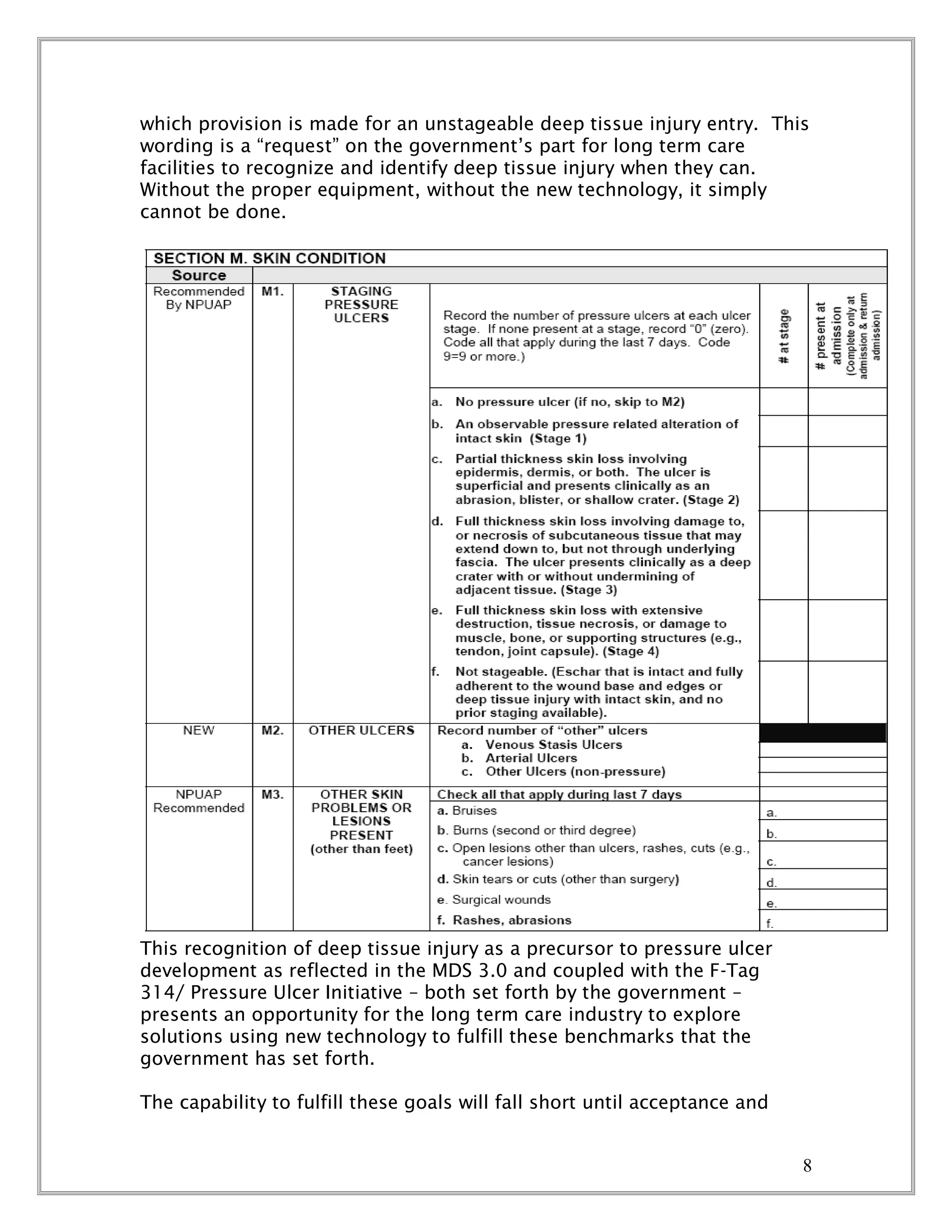
This document proposes a project called the Innovative Technology Application Project (ITAP) that aims to introduce new technologies to improve quality of life for long term care residents by better predicting, preventing, and treating pressure ulcers. The proposal seeks funding from the Center for Medicare and Medicaid Services to pilot these technologies in over 20 nursing homes for one year. It outlines the high costs and prevalence of pressure ulcers, and how new technologies like ultrasound imaging and thermal imaging could help identify deep tissue injuries earlier and improve outcomes. The goal of the pilot is to demonstrate how these technologies can be effectively and cost-efficiently incorporated into long term care facilities.