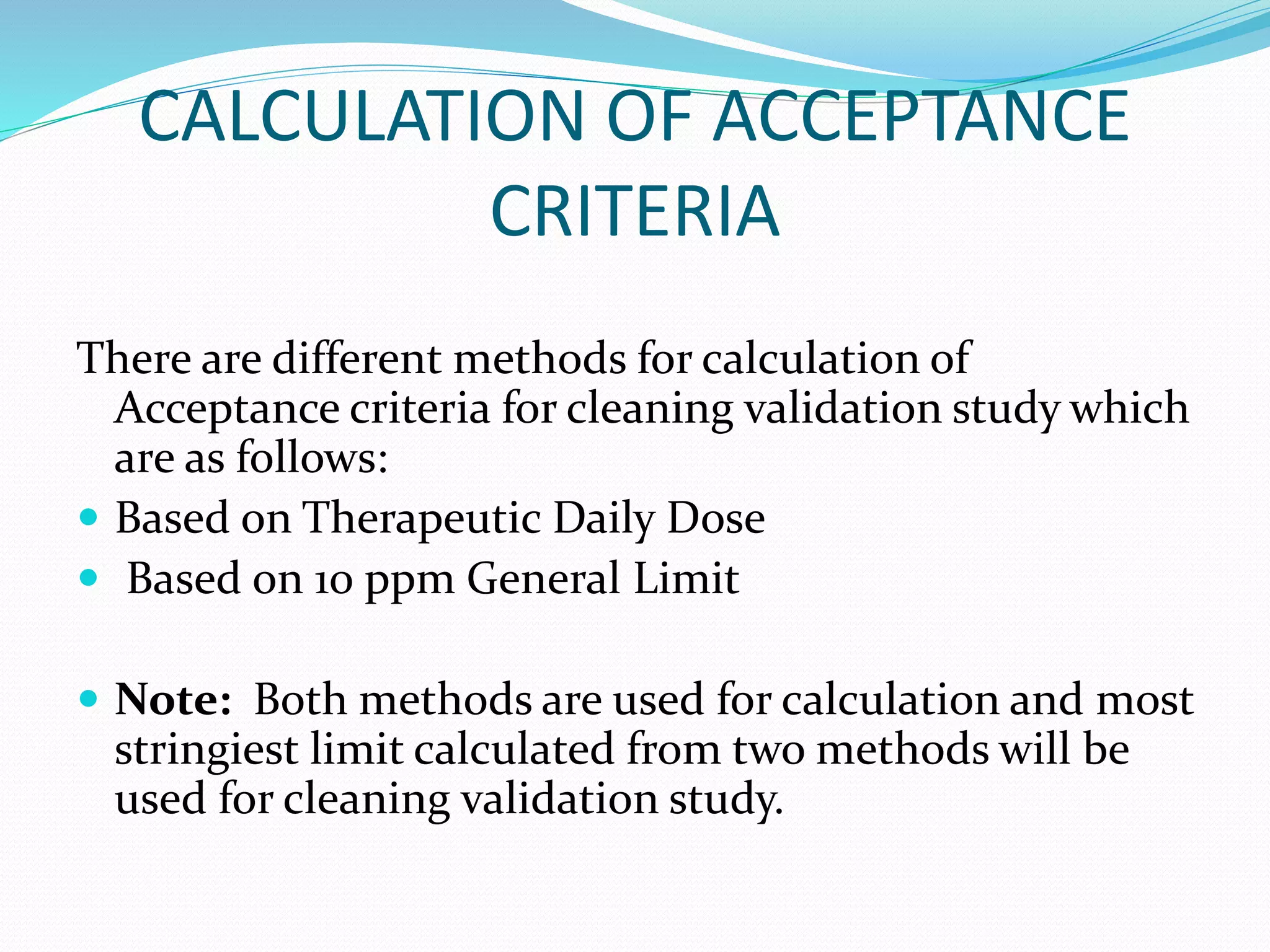
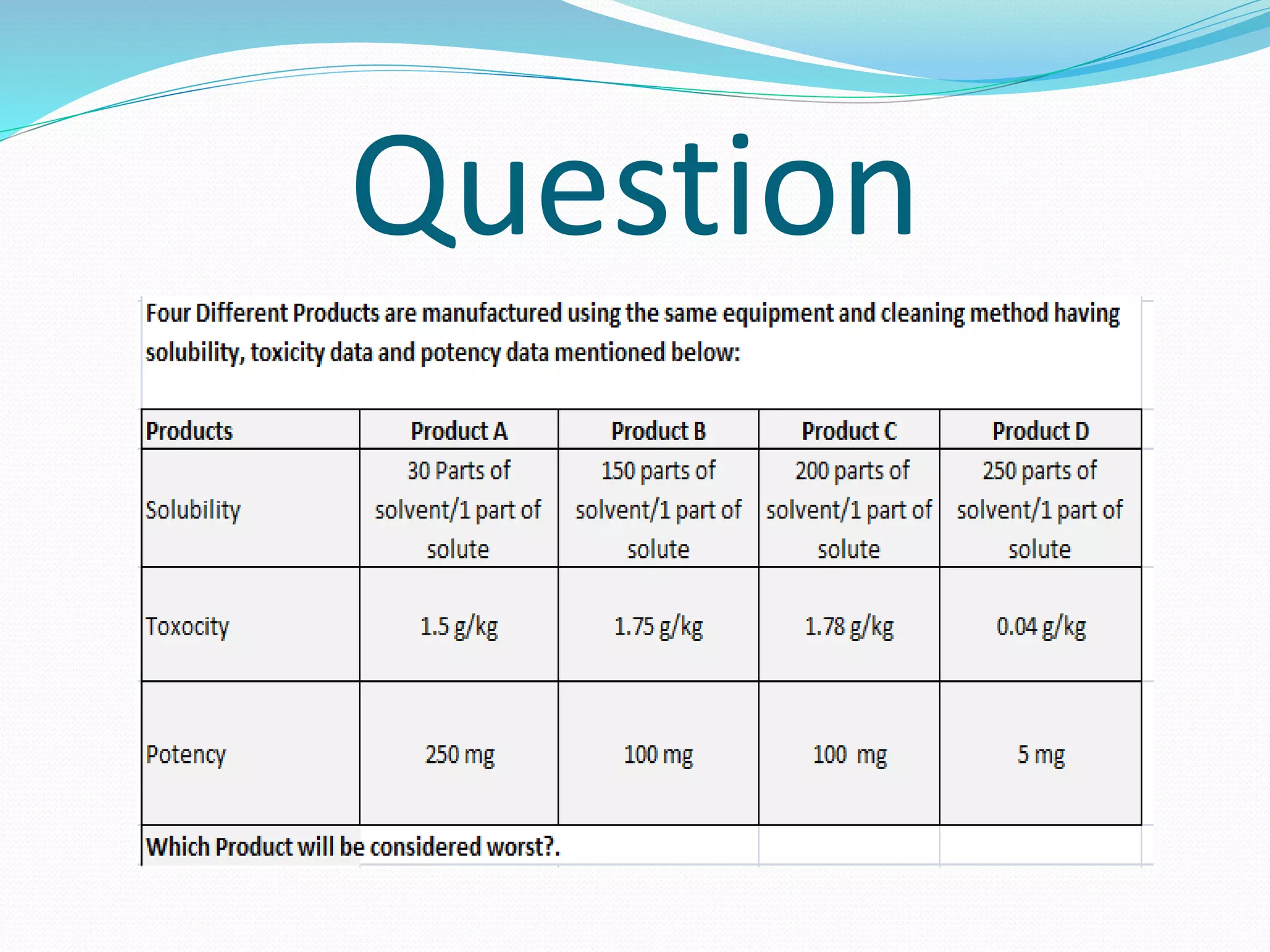
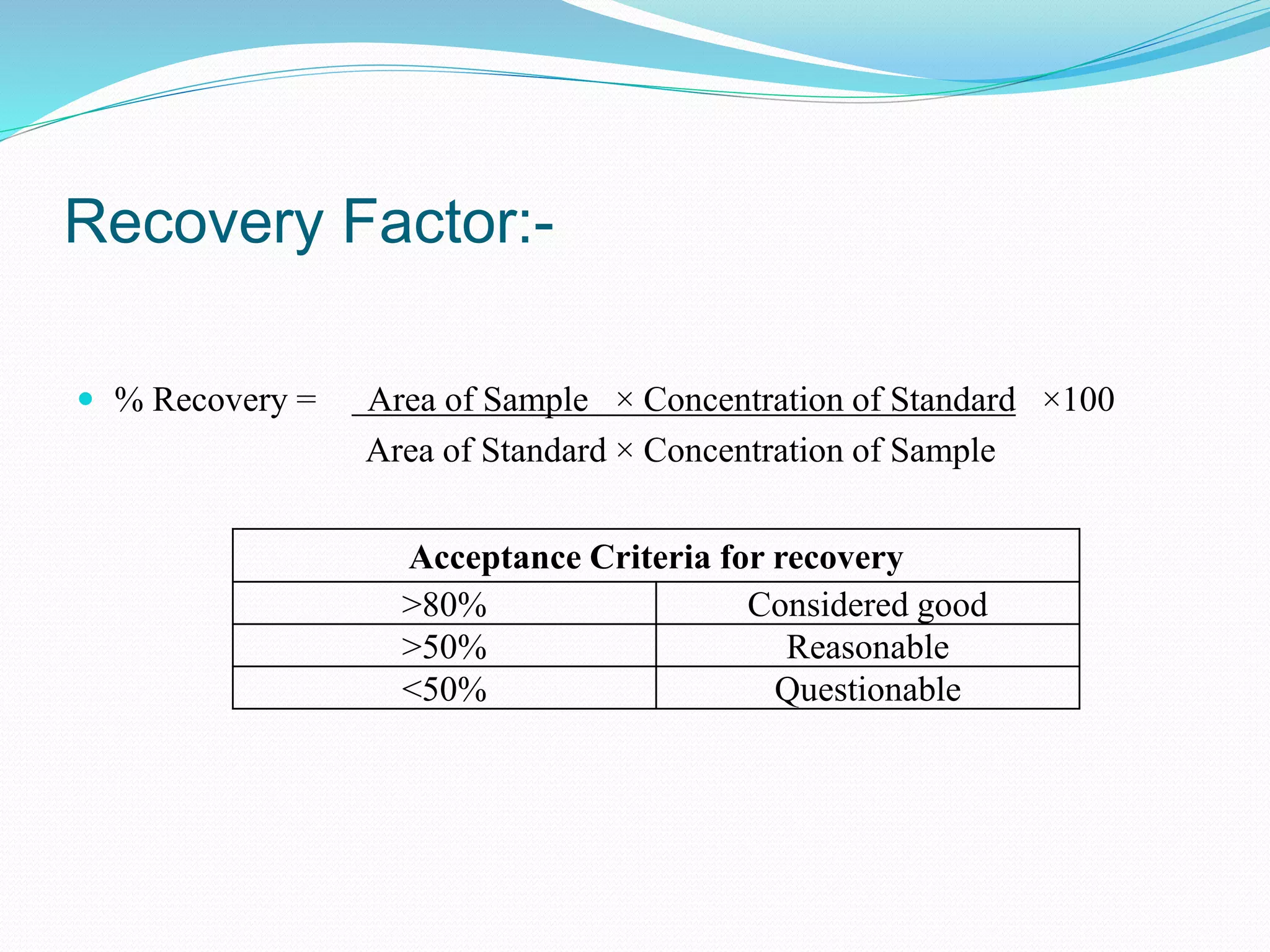
This document discusses cleaning validation, which is documented evidence that cleaning processes can effectively and reproducibly clean equipment to a predefined acceptable level. It outlines the objectives of cleaning validation to verify that equipment is consistently cleaned of residues to an acceptable level. Acceptance criteria are typically calculated using therapeutic daily dose or a 10 ppm general limit. Recovery studies determine the percentage of residues that can be recovered from equipment surfaces. Sampling, equipment hold times, cleaning process parameters, and routine monitoring are also discussed.