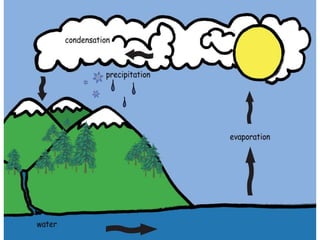
Physical change involves altering the appearance, form, shape, or size of matter without changing its composition or transforming it into a new substance. Matter can change states between solid, liquid, and gas through various processes including melting, evaporation, condensation, solidification, and sublimation. These state changes are examples of physical changes and occur when matter gains or loses energy, causing the particles that make up the matter to move differently.