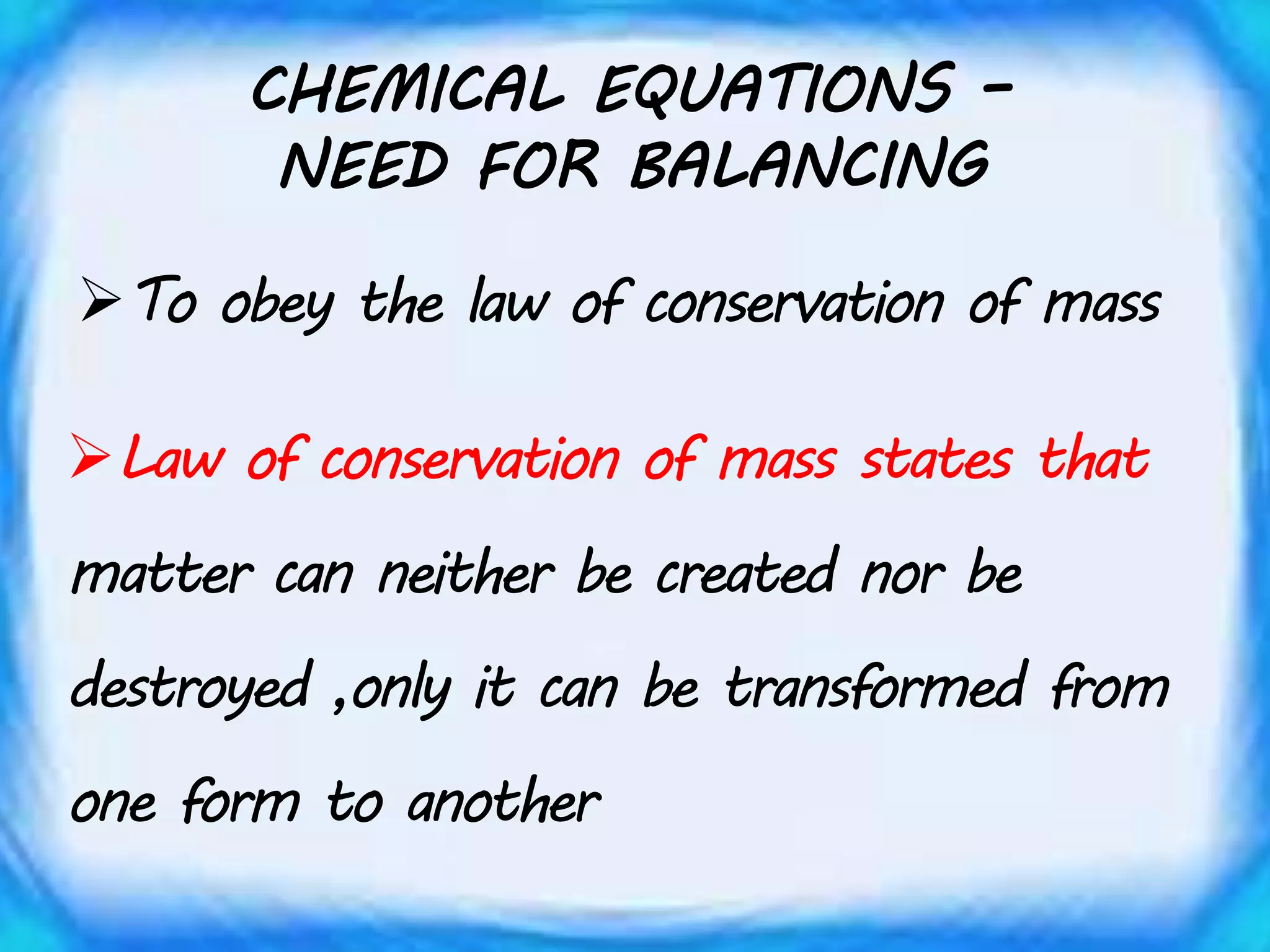
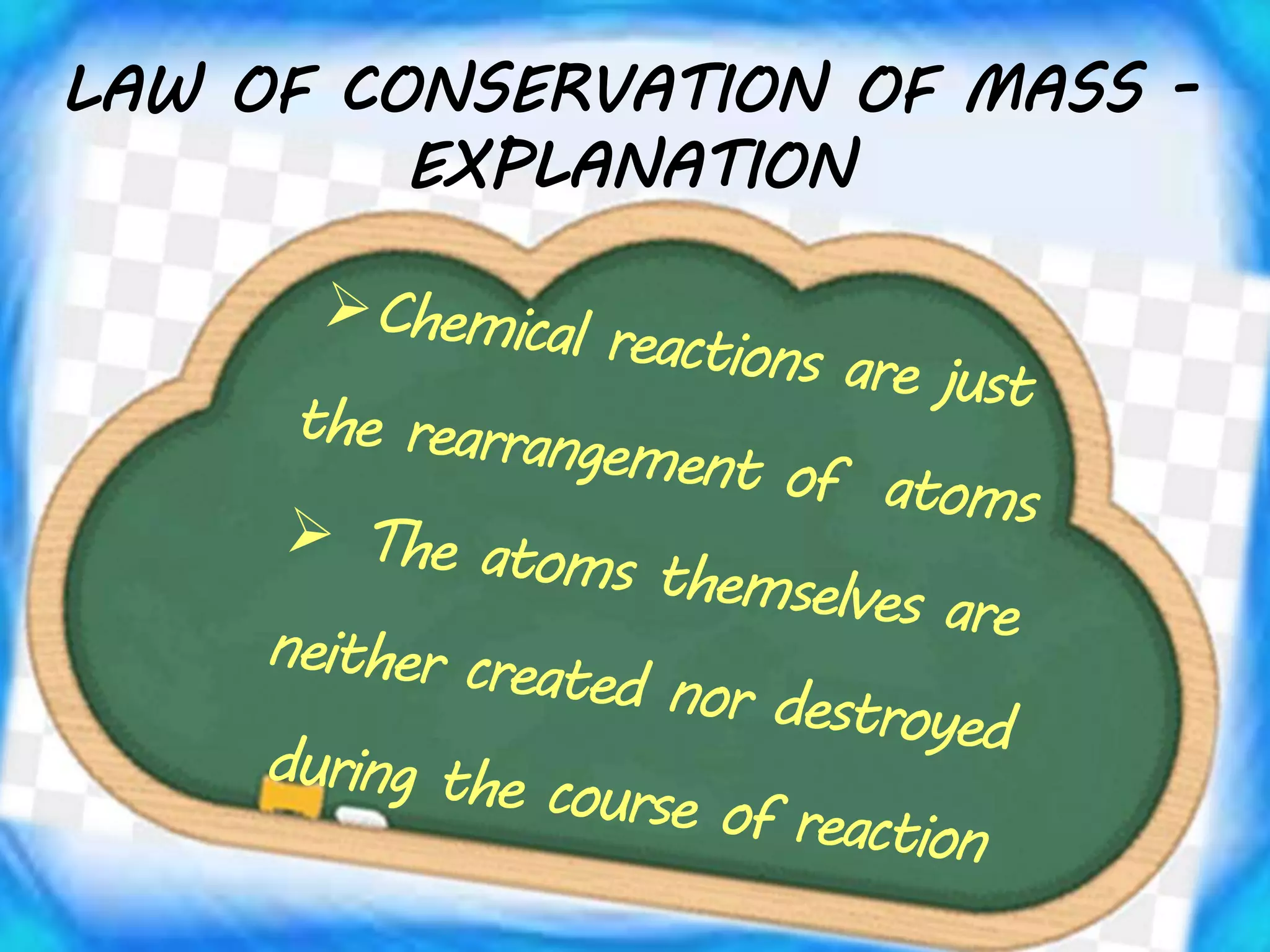
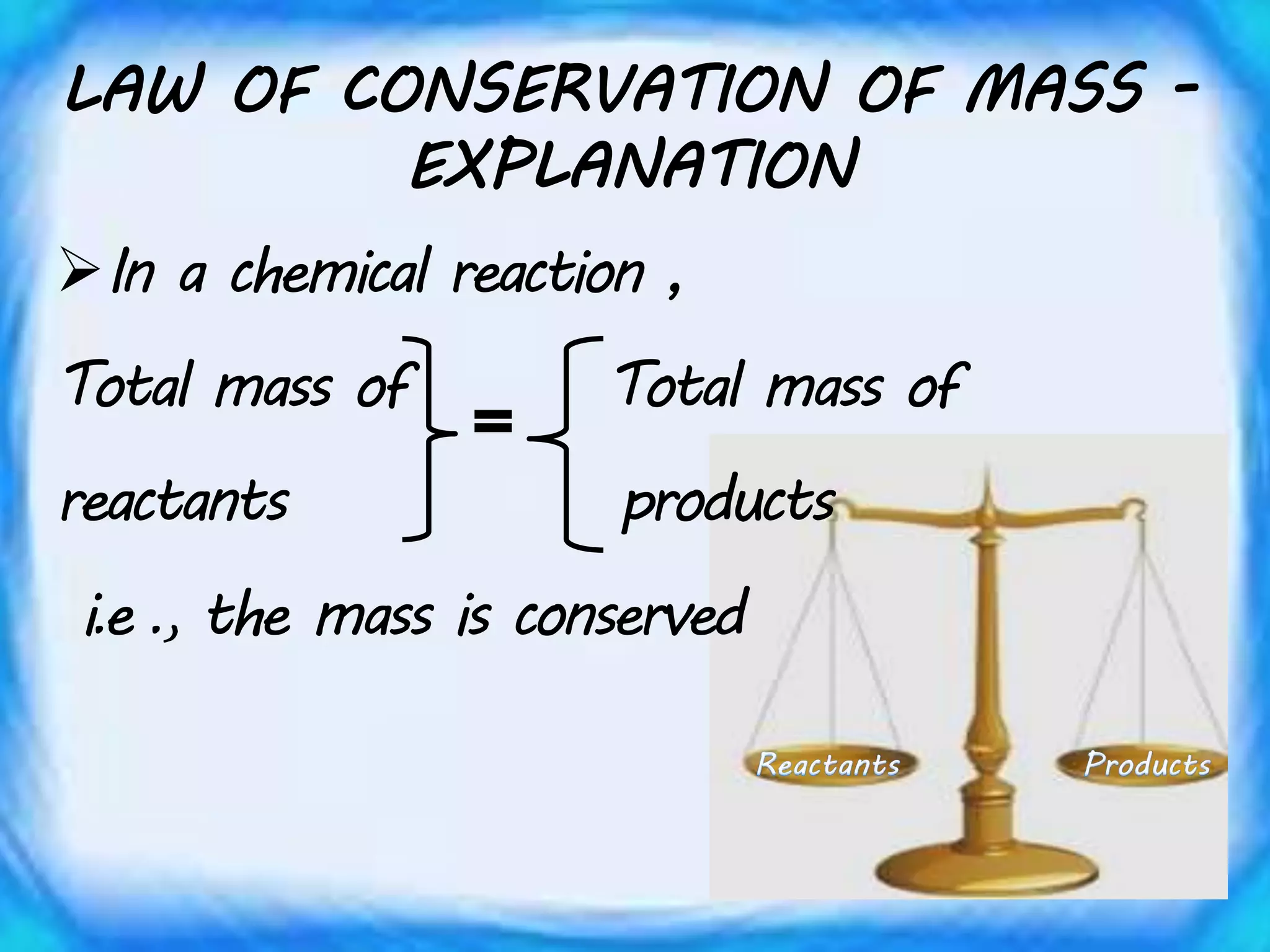
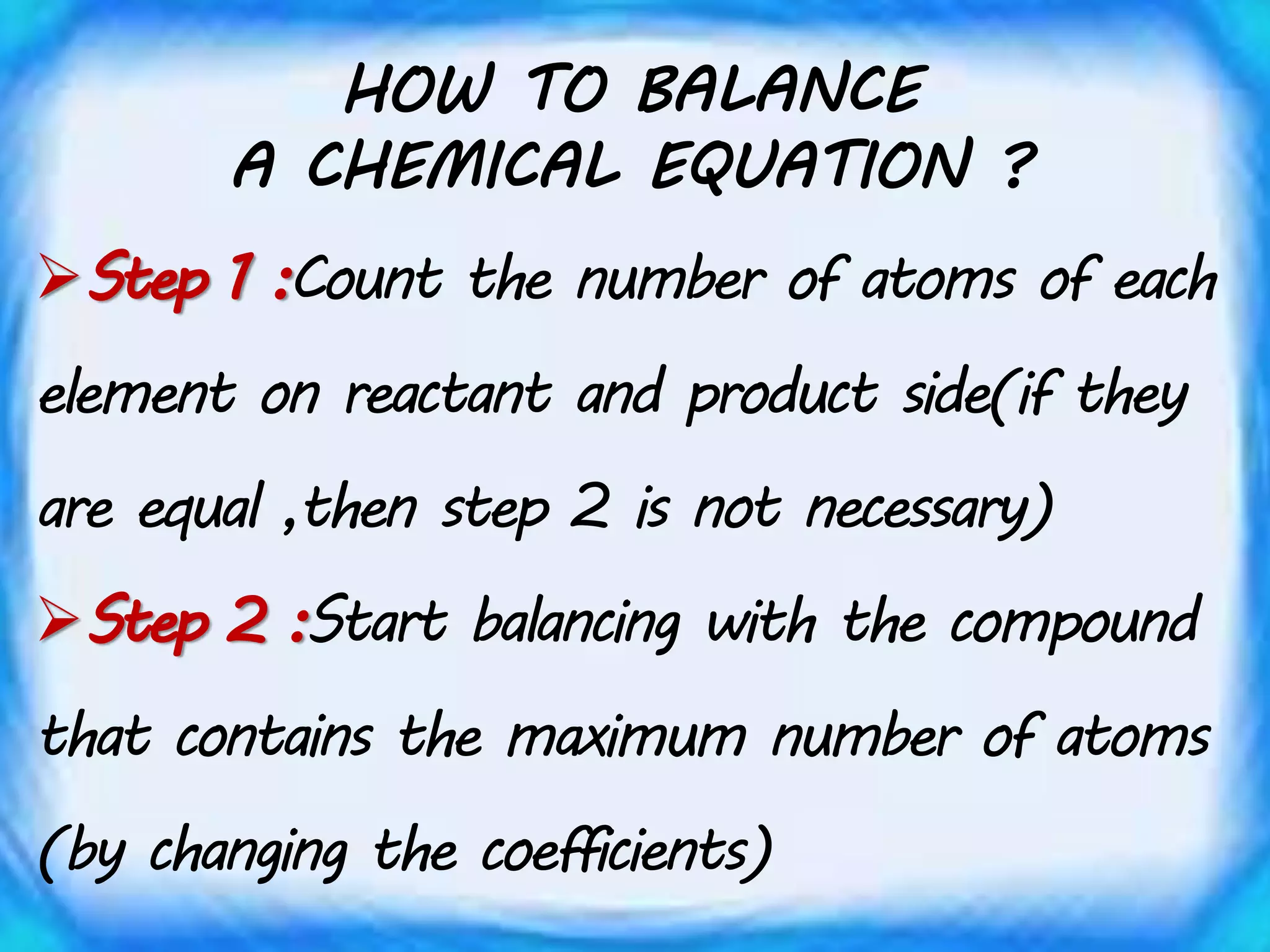
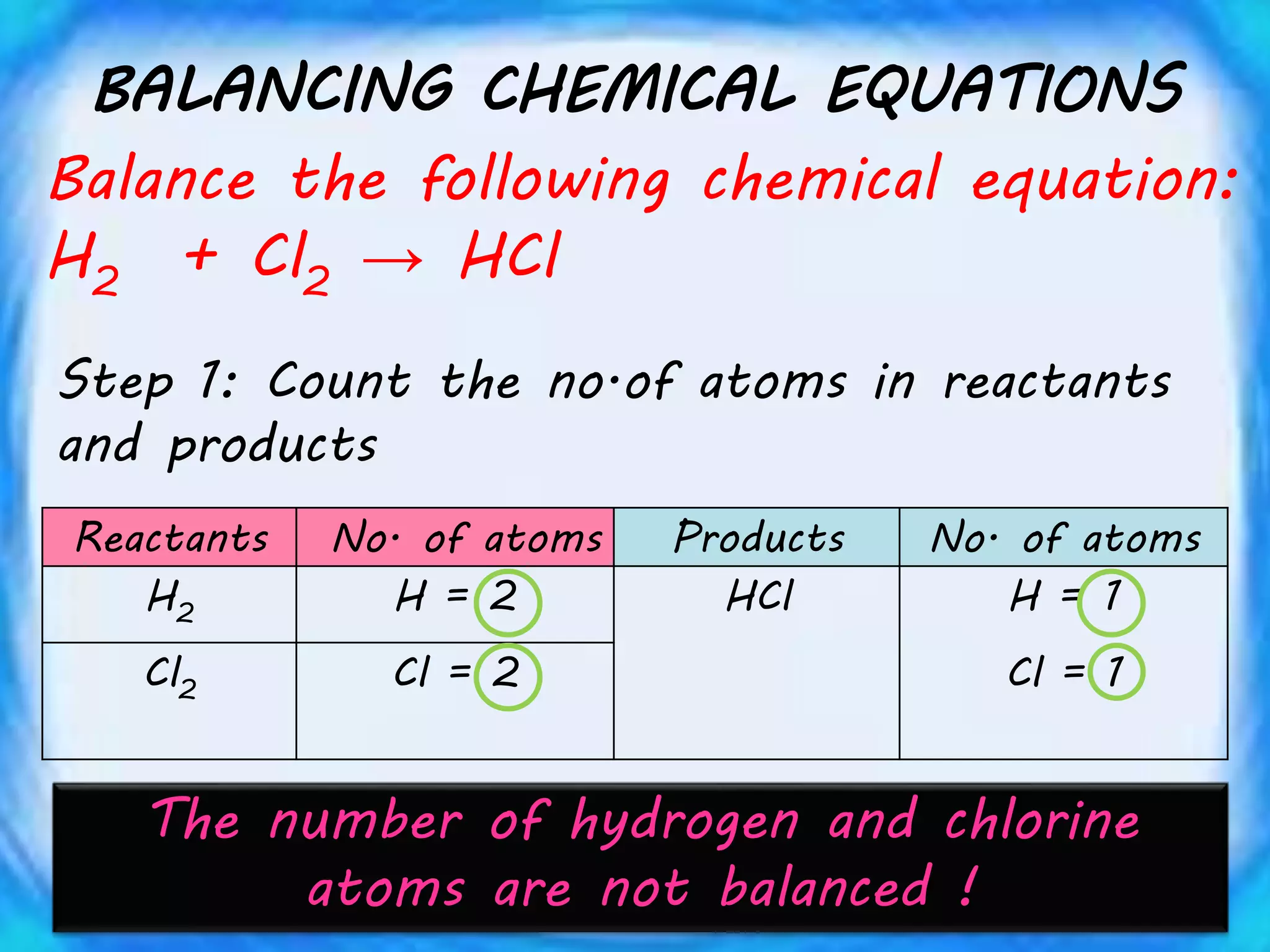
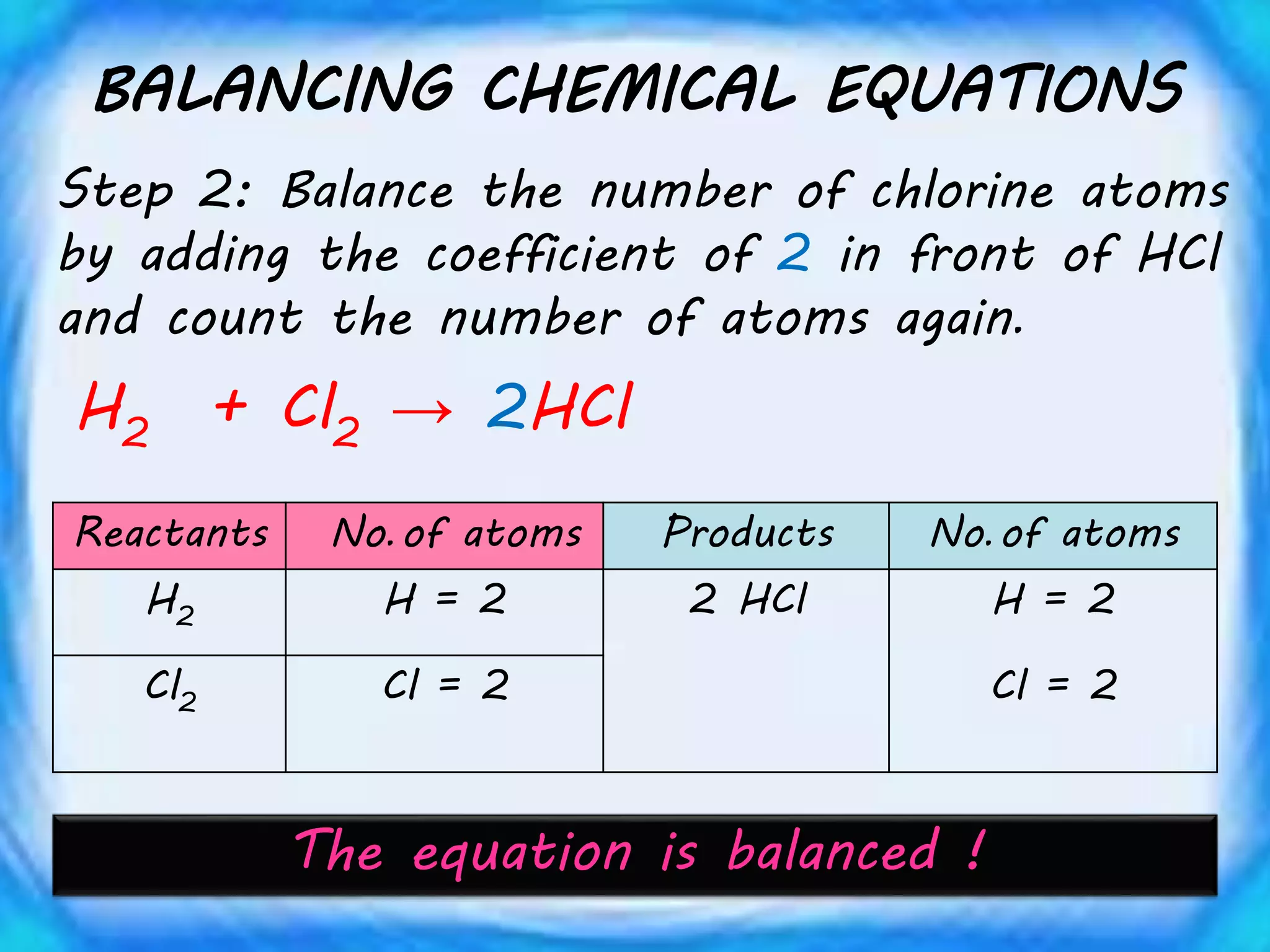
Chemical equations must be balanced to obey the law of conservation of mass. This law states that matter is neither created nor destroyed in a chemical reaction, only transformed. To balance an equation, one counts the number of atoms of each element on both sides of the reaction. If the numbers are not equal, coefficients are placed in front of formulas to balance the atoms. For example, the equation H2 + Cl2 → HCl is balanced by adding a coefficient of 2 in front of HCl to make the chlorine atoms equal on both sides of the reaction. Balancing chemical equations ensures the law of conservation of mass is followed.