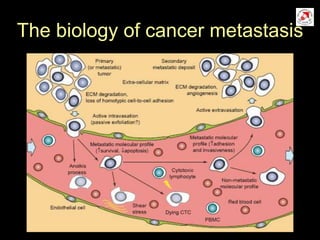
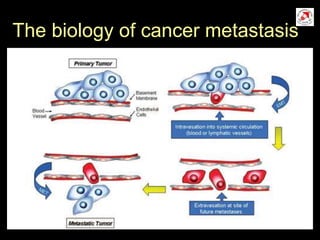
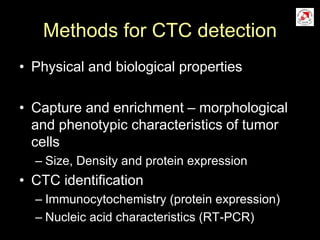
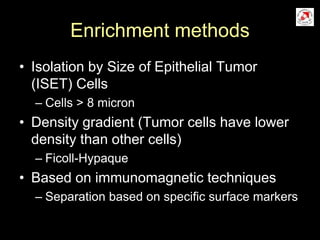
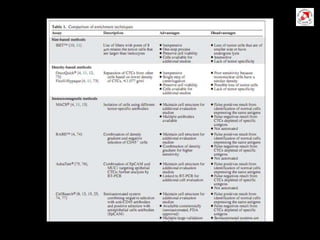
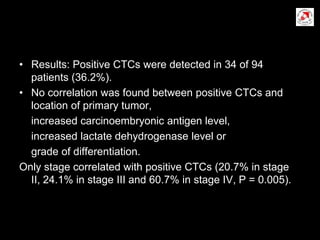
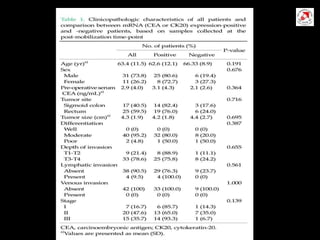
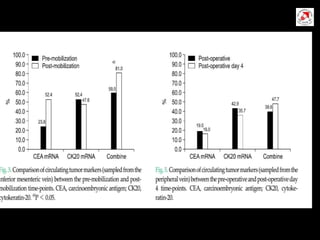
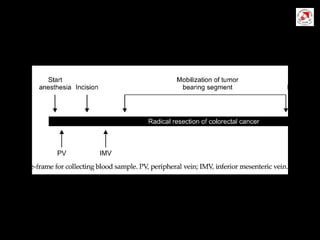
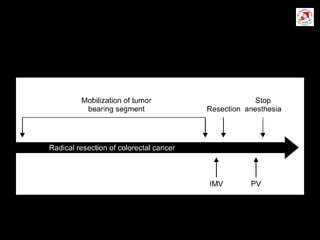
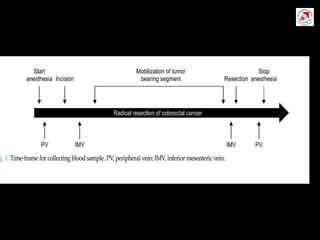
Circulating tumor cells (CTCs) have potential clinical applications as biomarkers in colorectal cancer. Studies have found CTCs correlate with disease stage but not other clinical factors. Detecting CTCs before and during treatment can independently predict progression-free and overall survival. While CTC detection provides prognostic information, methodology challenges remain around isolating, quantifying, and characterizing CTCs reproducibly. Further research could help validate CTCs against standard biomarkers and guide personalized therapy.