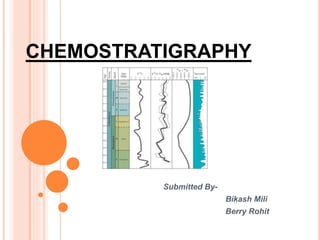
Chemostratigraphy is the study of chemical variations in rock sequences based on elemental or isotopic composition. It provides a useful tool for unconventional resource exploration. Some chemical signatures in rocks, like stable isotopes, can be used as accurate stratigraphic markers because they record global environmental changes over thousands of years. Common tools in chemostratigraphy include oxygen, carbon, and strontium isotope analysis, with strontium isotopes widely used for correlation due to long ocean residence times allowing identification of major shifts in the isotopic curve.