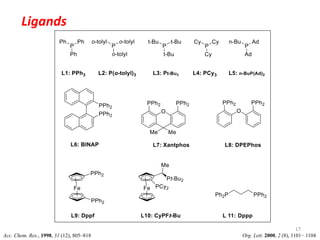
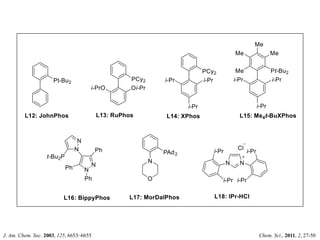
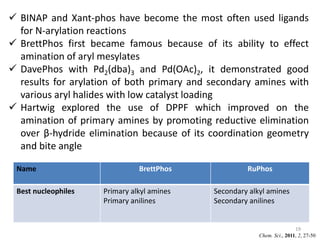
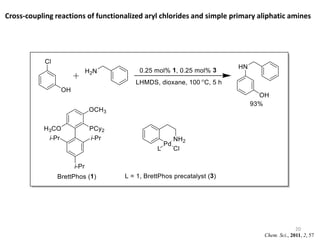
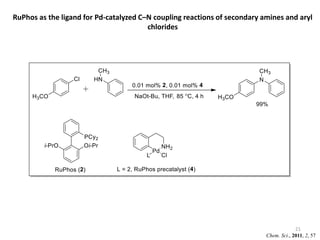
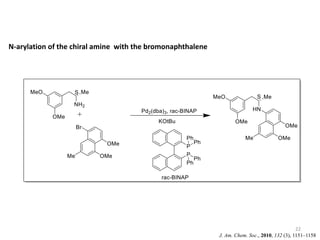
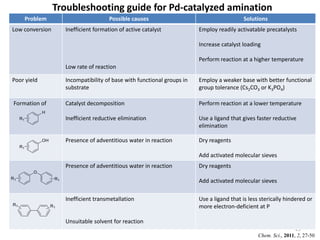
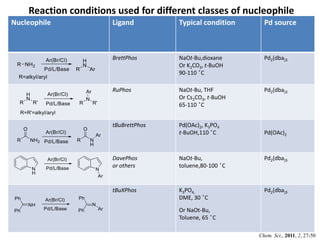
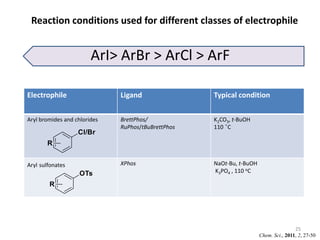
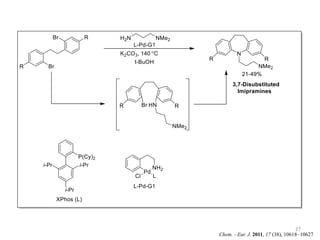
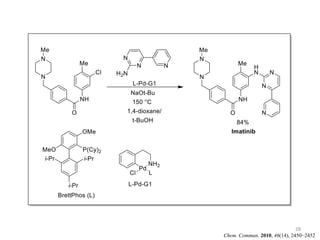
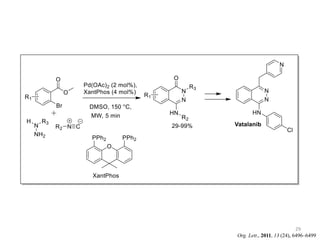
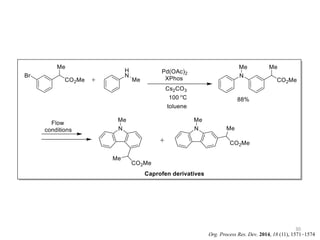
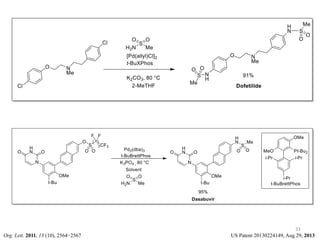
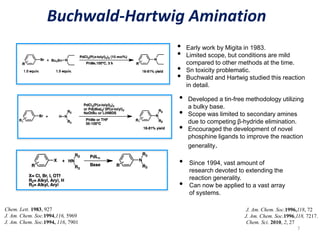
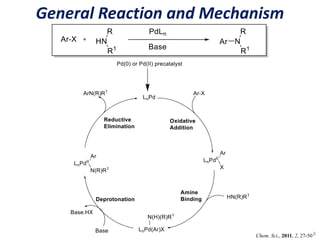
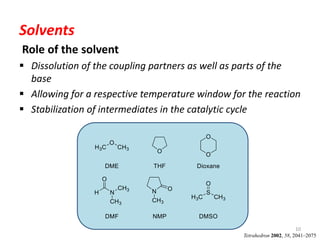
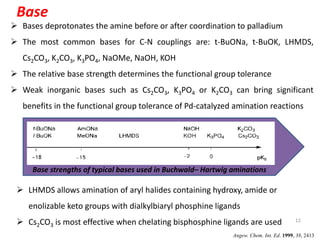
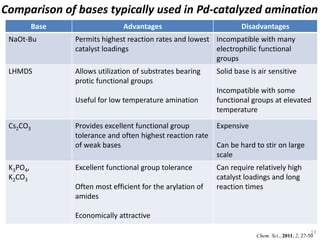
This document discusses palladium-catalyzed Buchwald-Hartwig C-N coupling reactions and their applications in medicinal chemistry. It provides an overview of the reaction mechanism and components of the catalytic system, including solvents, bases, palladium sources, and ligands. Common troubleshooting issues are also reviewed, such as low conversion or yield that can be addressed by optimizing reaction conditions like temperature, base selection, drying of reagents, and ligand choice to promote reductive elimination. Examples are given of applications in coupling secondary amines and functionalized substrates. In summary, Buchwald-Hartwig amination is a versatile method for C-N bond formation widely used in drug development.


![14
Palladium
Typically, Pd(0) or Pd(II) precursors are used
The most prominent Pd(0) precursors are Pd2(dba)3 and Pd(dba)2
The most versatile Pd(II) precursor is Pd(OAc)2. [allPdCl]2 or Pd(acac)2 also
show remarkable activity in special cases
One of the most abundant Pd(II) salts is PdCl2, efficient in the amination of
aryl bromides using diphosphines but not very promising with mono-
phosphines
The palladium catalyst must be in the (0) oxidation state before the catalytic
cycle initiates, and therefore the palladium(II) in Pd(OAc)2 must be reduced
prior to catalysis initiation
Can. J. Chem. , 2001, 79(11), 1799-1805](https://image.slidesharecdn.com/chemo-210713072340/85/Chemo-14-320.jpg)
![Pd2(dba)3
In the case of Pd2(dba)3 (dba = dibenzylideneacetone), the oxidation state
of palladium is already (0) and there is no need for reduction
In this case reaction with phosphines L gives species of the type Pd(dba)L2
rather than Pd(0)Ln
15
Pd(OAc)2
Pd(OAc)2 is usually reduced to palladium(0) complex [Pd(OAc)L2]- by
phosphines
At least 3 equivalents of PPh3 is needed for the reduction of Pd(OAc)2
Chem. Sci., 2011, 2, 27-50](https://image.slidesharecdn.com/chemo-210713072340/85/Chemo-15-320.jpg)