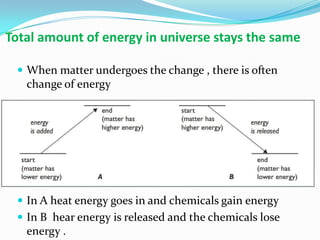
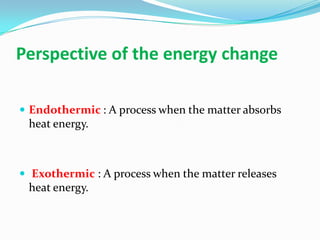
1) Chemical reactions can be endothermic or exothermic depending on whether heat energy is absorbed or released during the reaction.
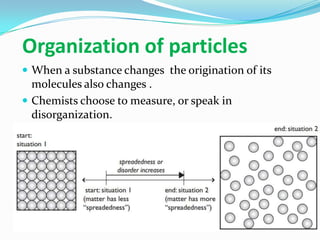
2) The organization and disorder of particles changes as matter changes form - gases have more disorder than solids or liquids.
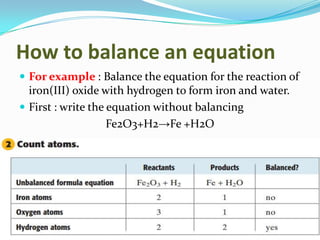
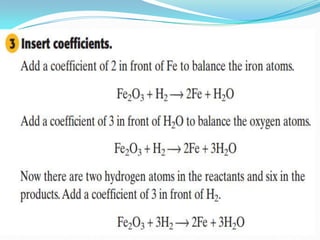
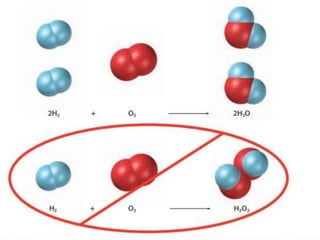
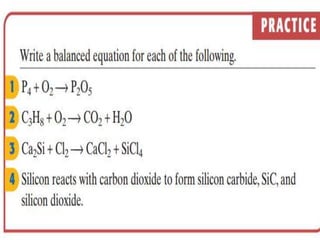
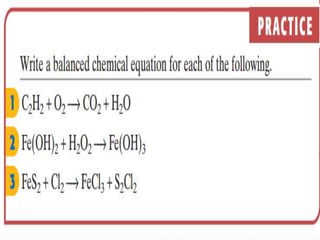
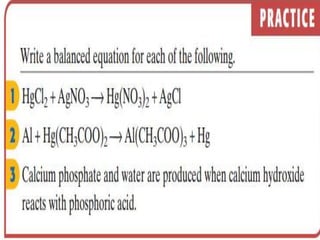
3) Balanced chemical equations follow the law of conservation of matter, with the same number and type of atoms on both sides of the equation.