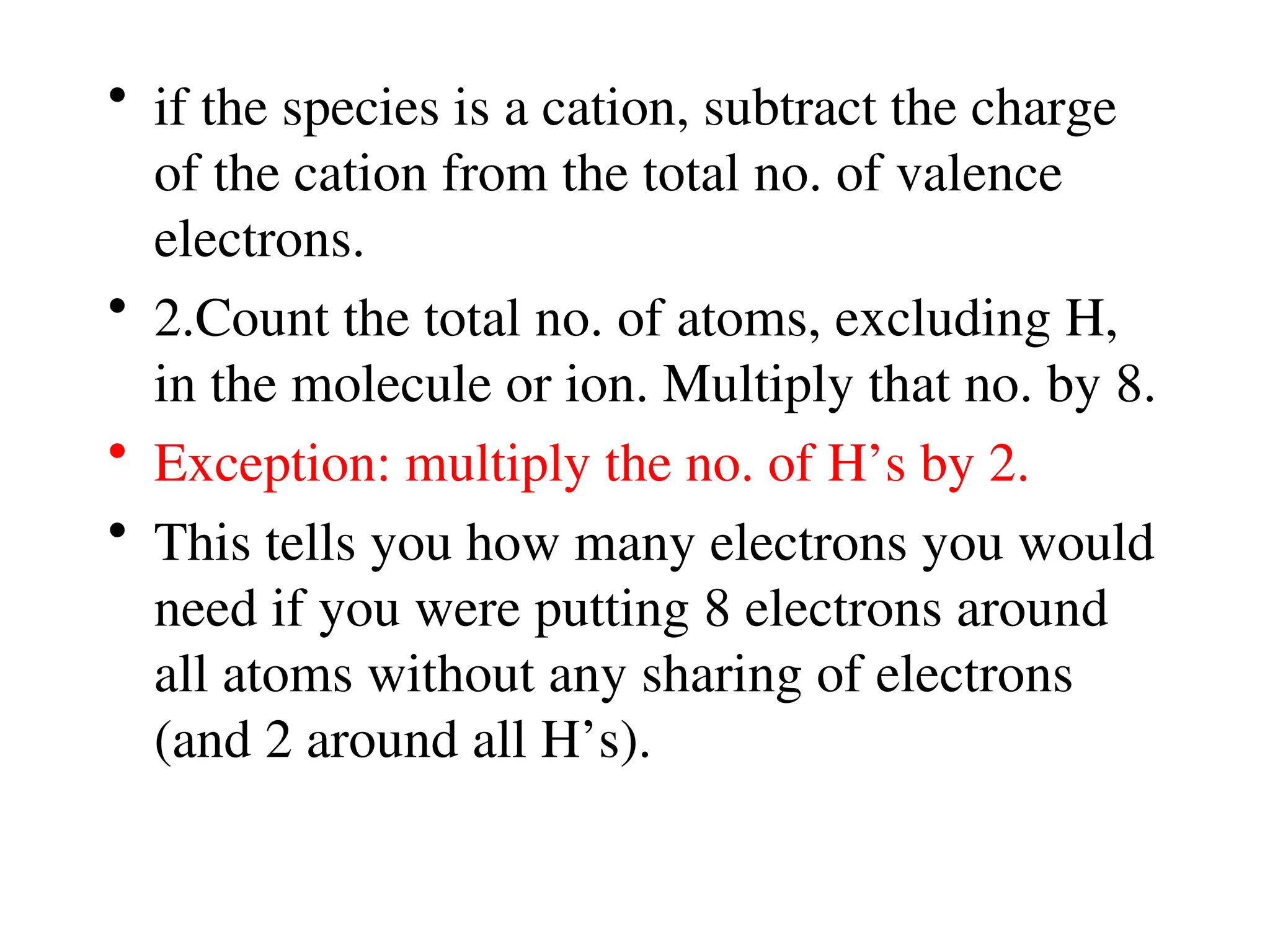
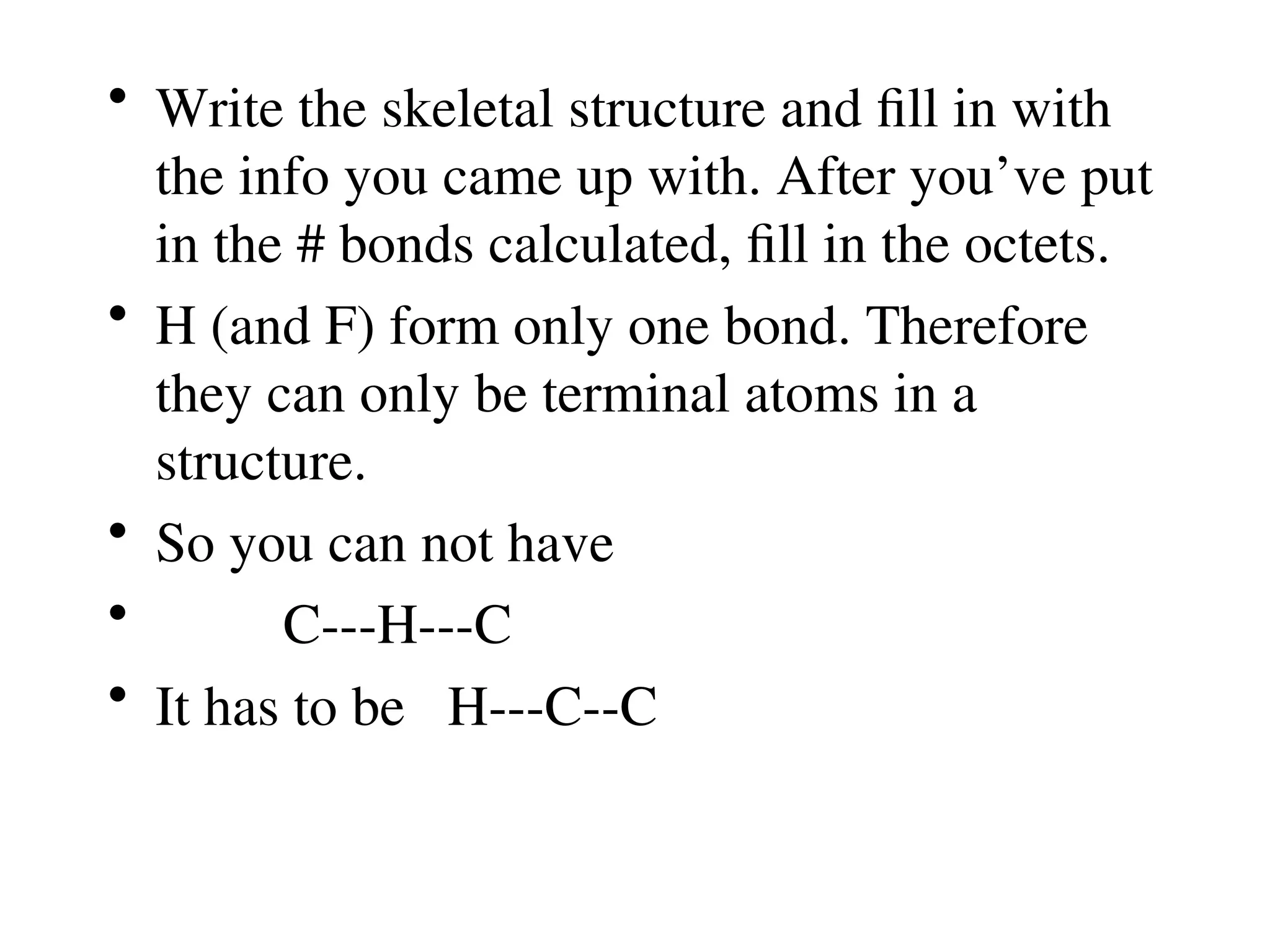
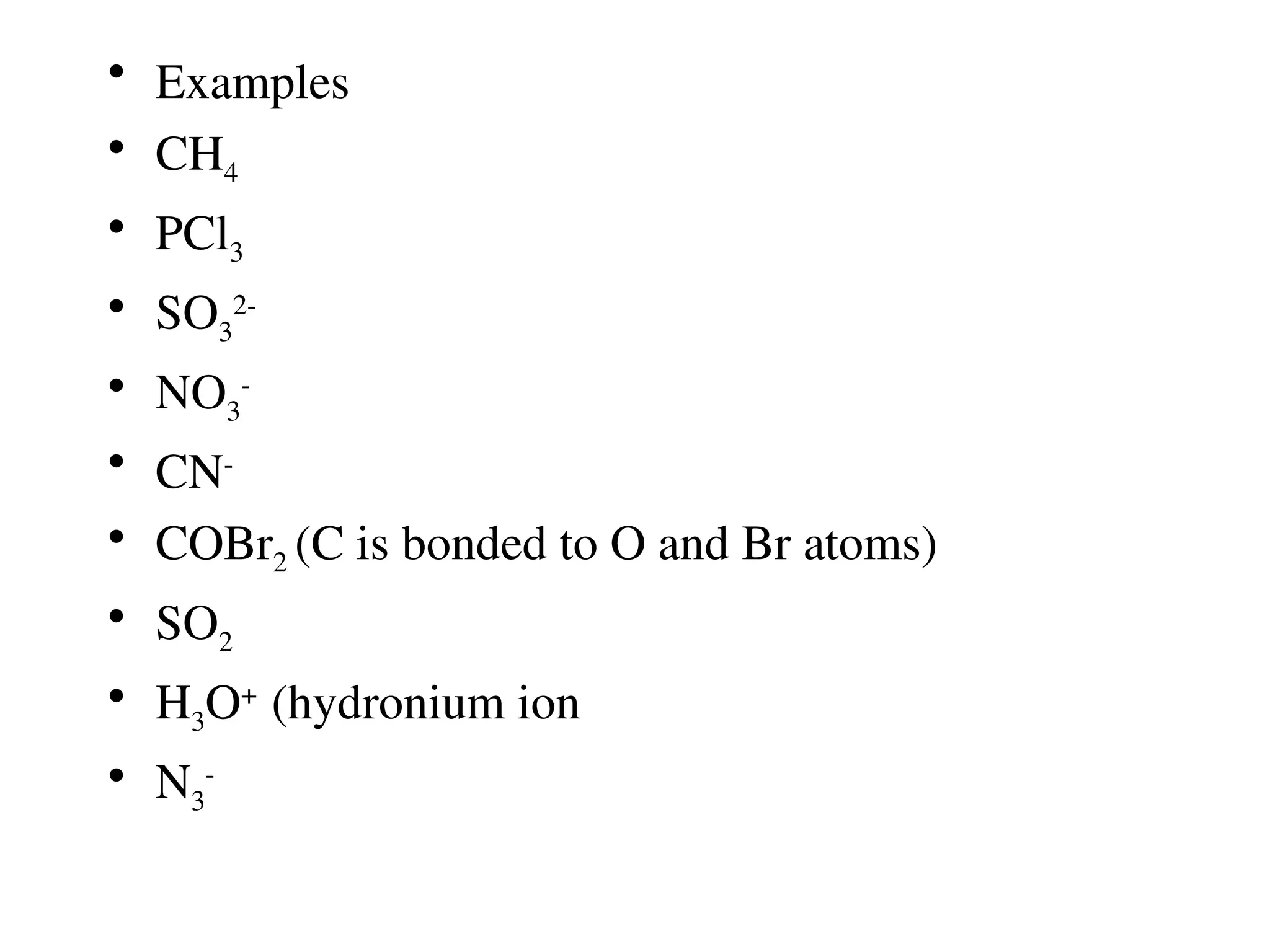
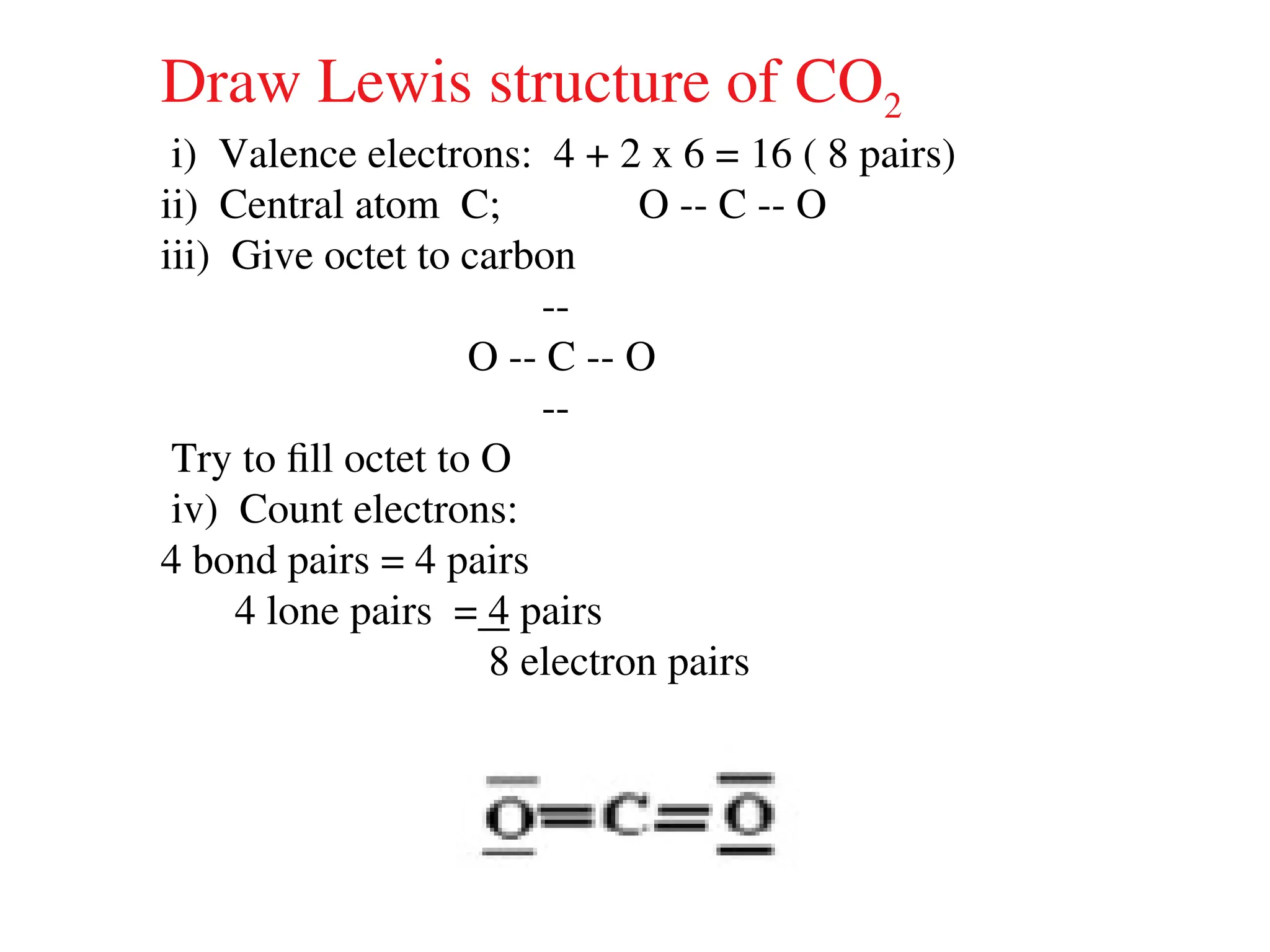
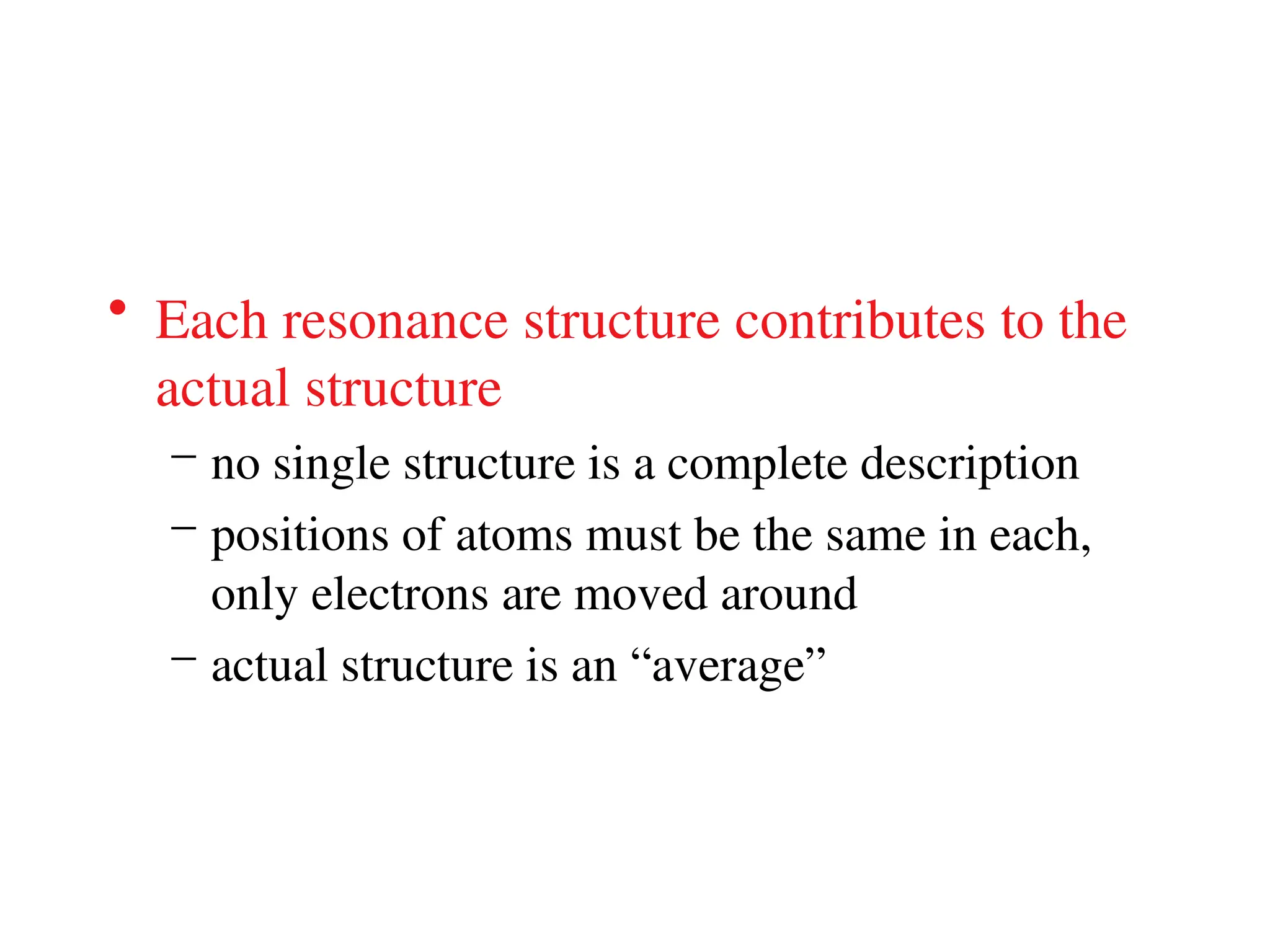
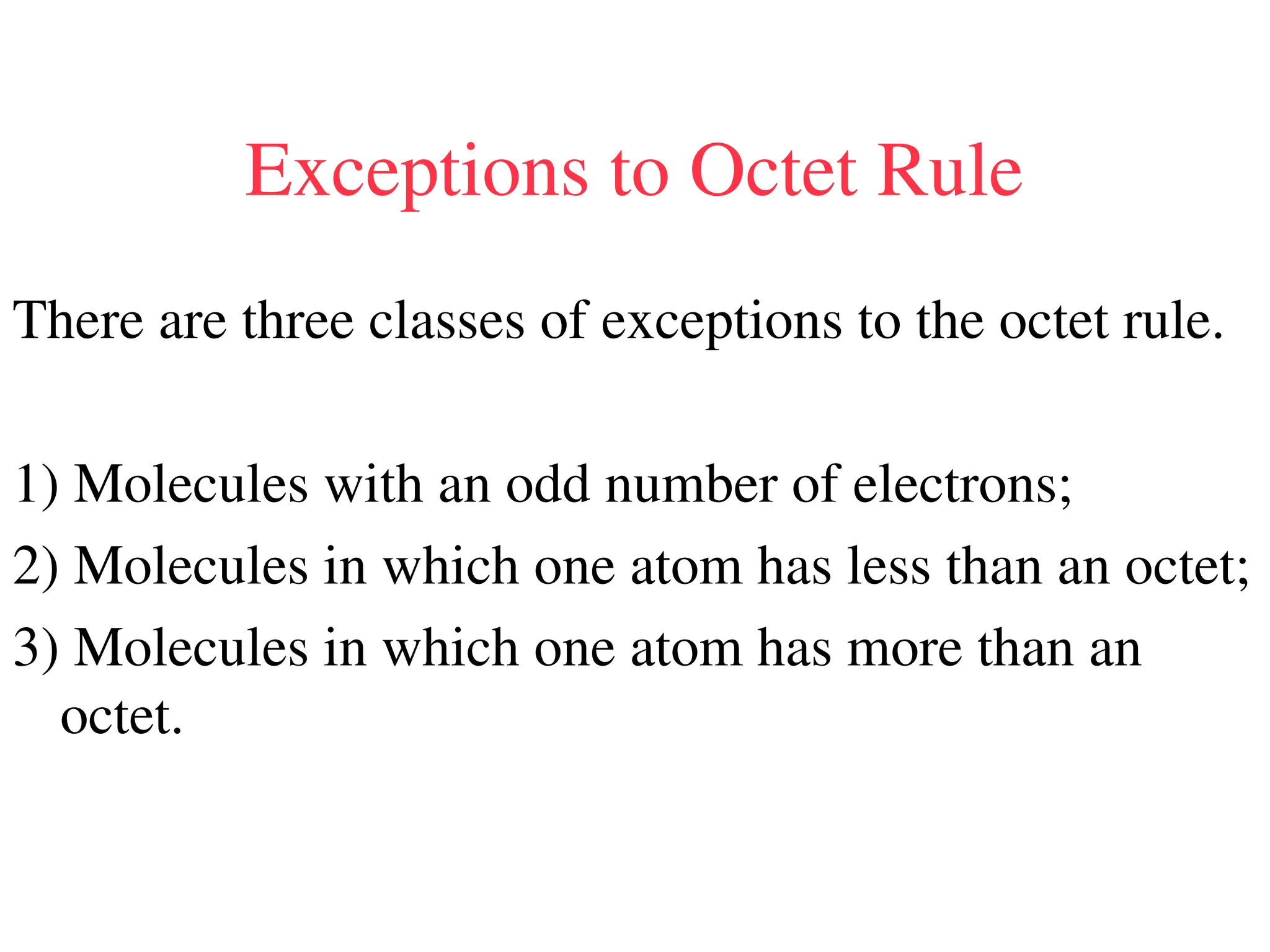
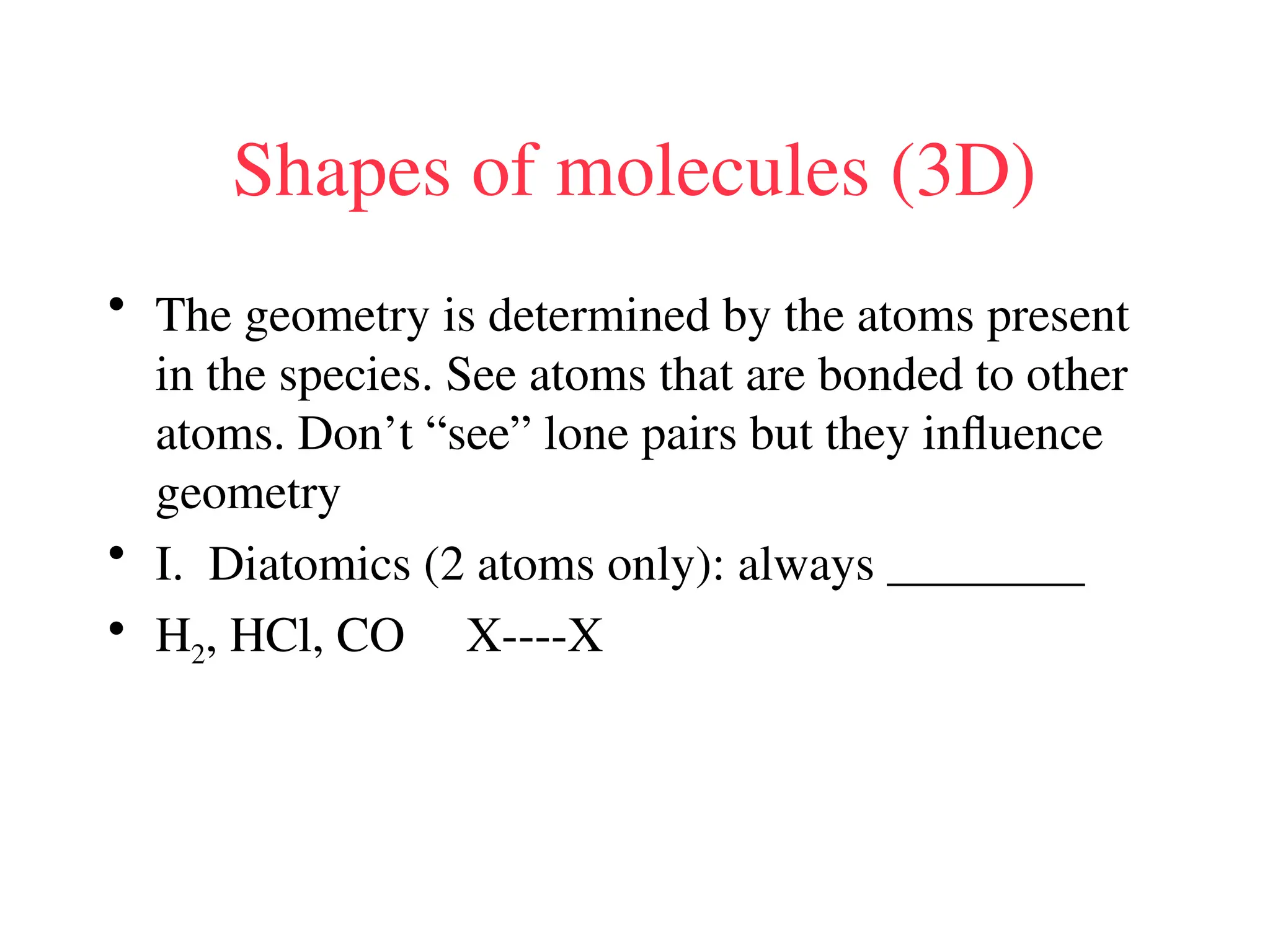
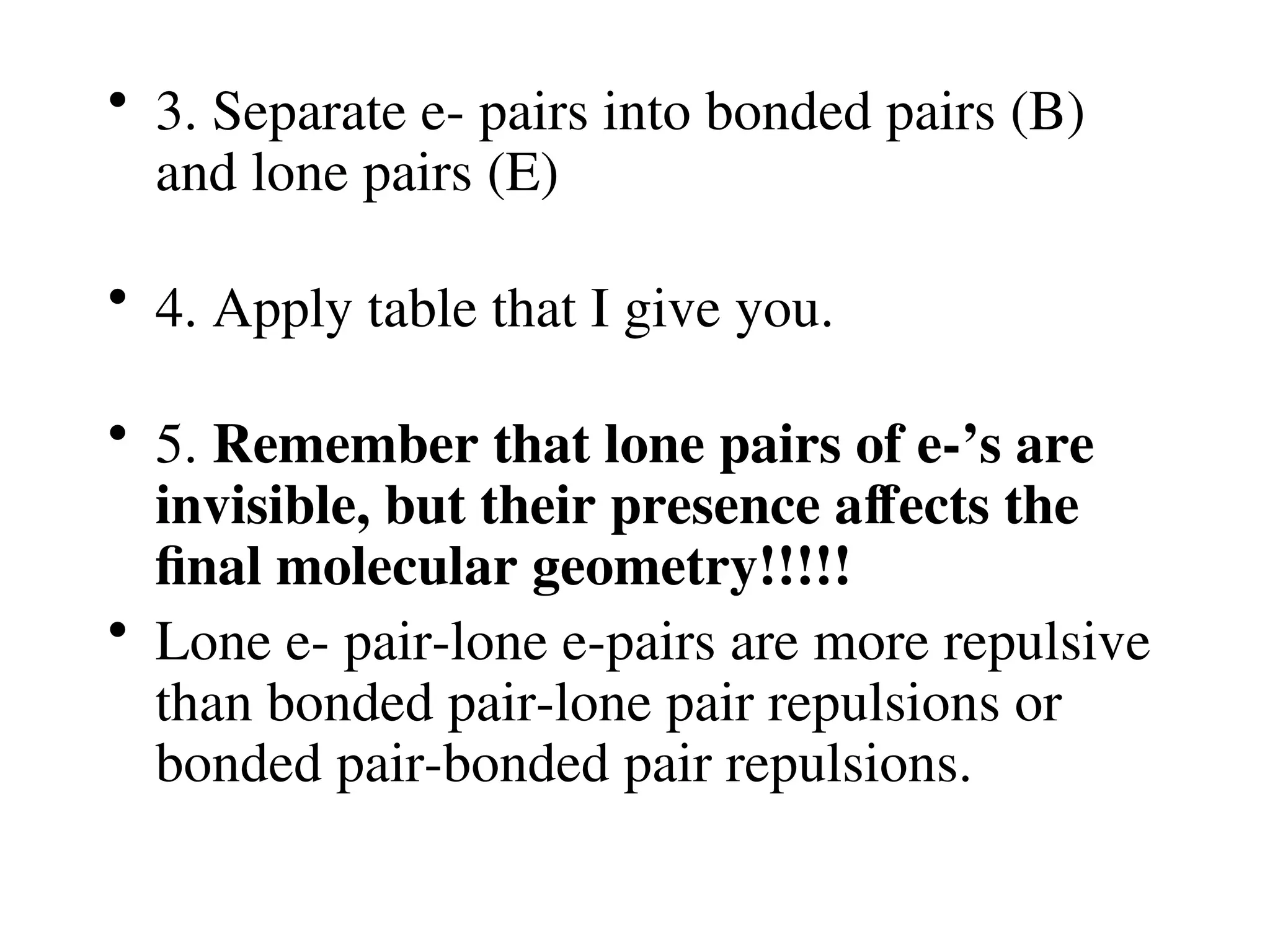
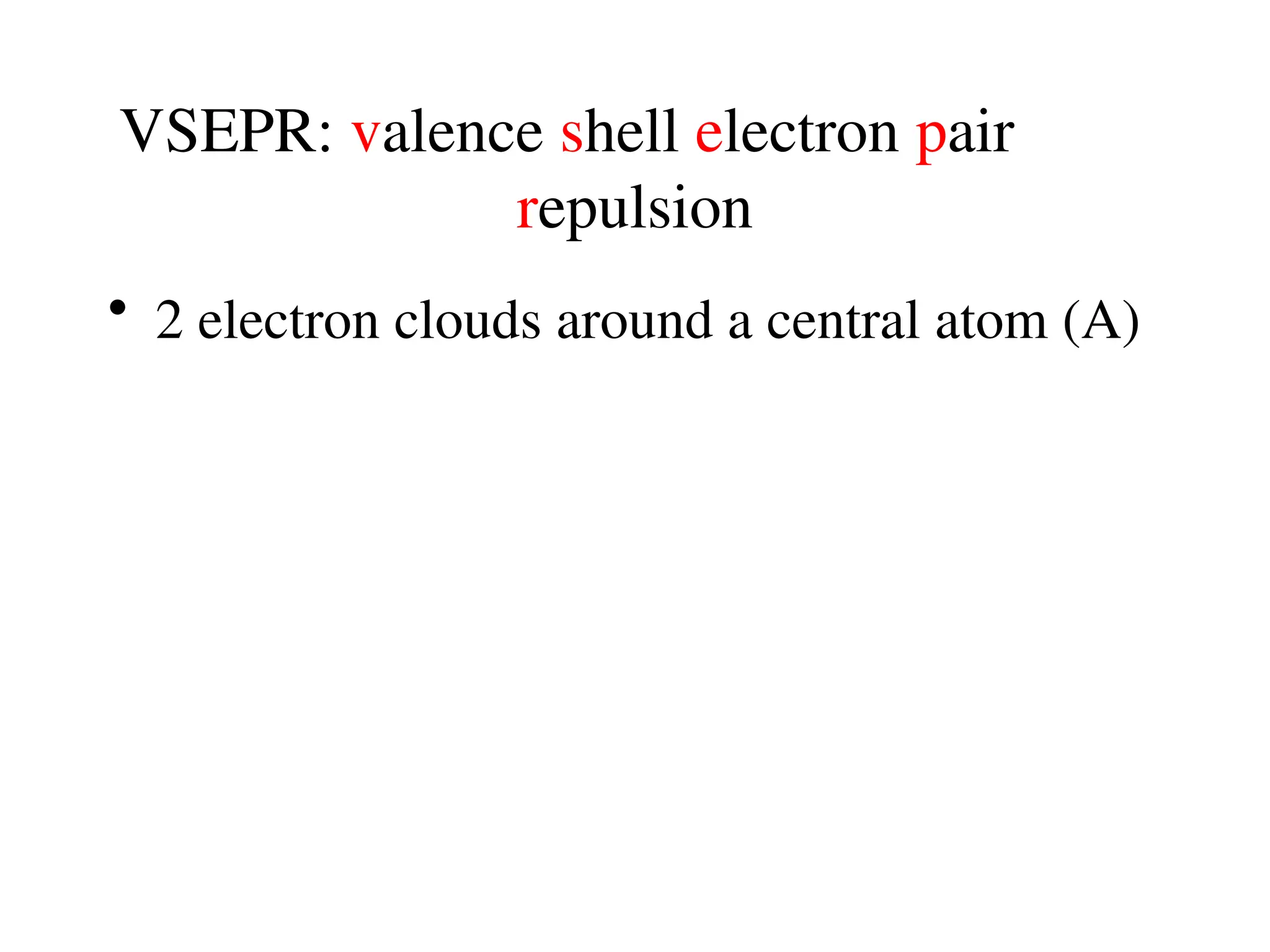
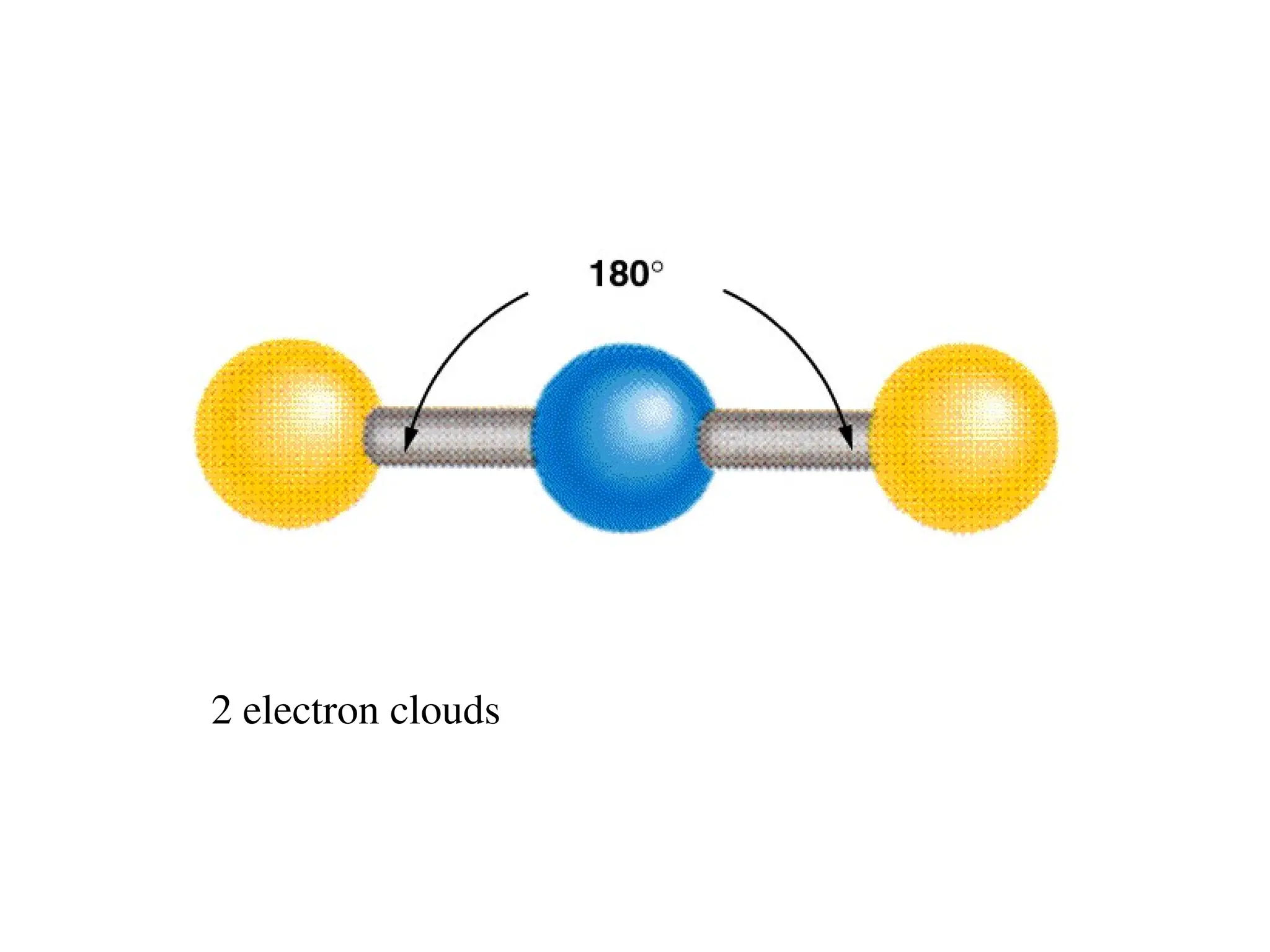
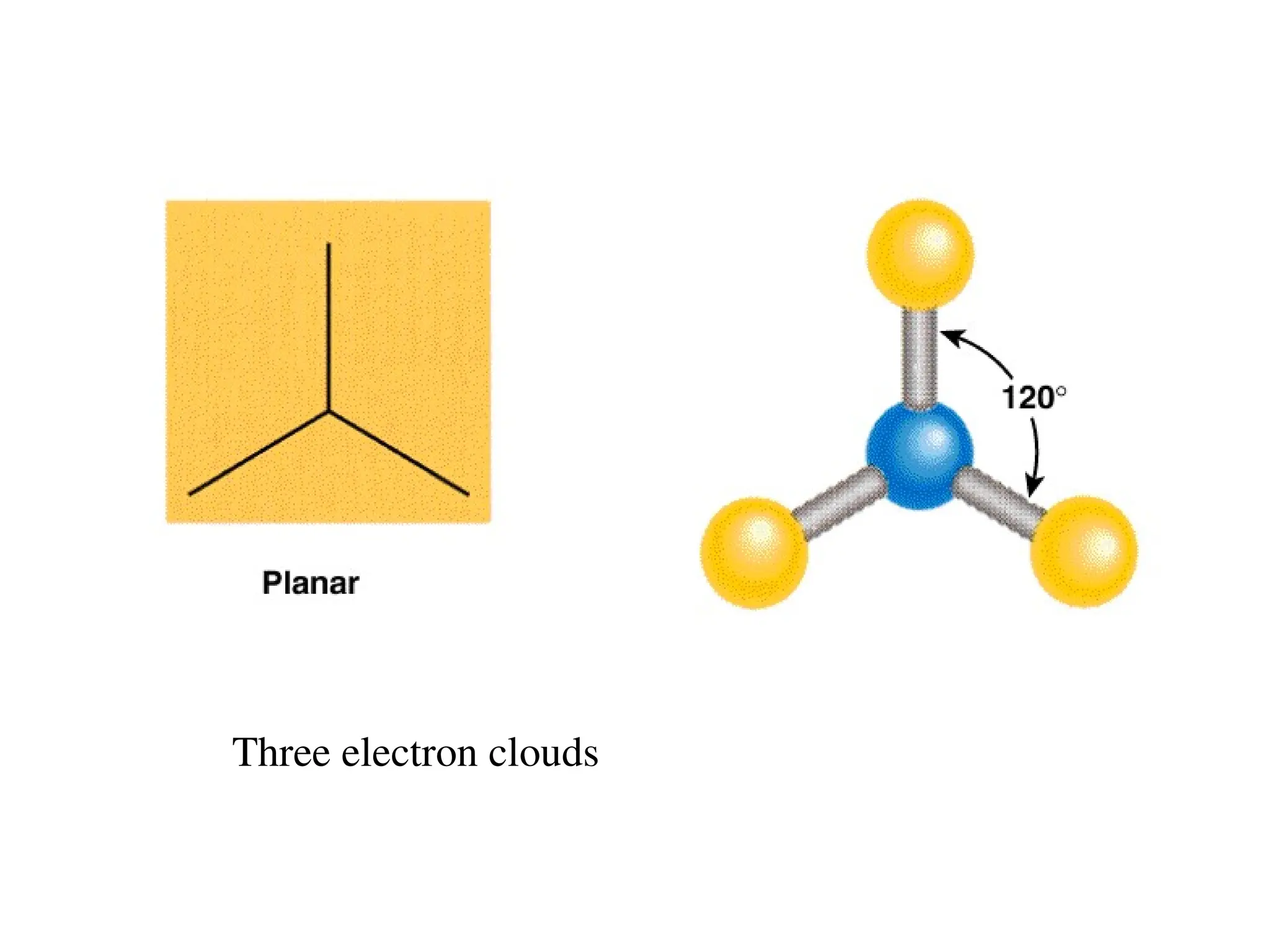
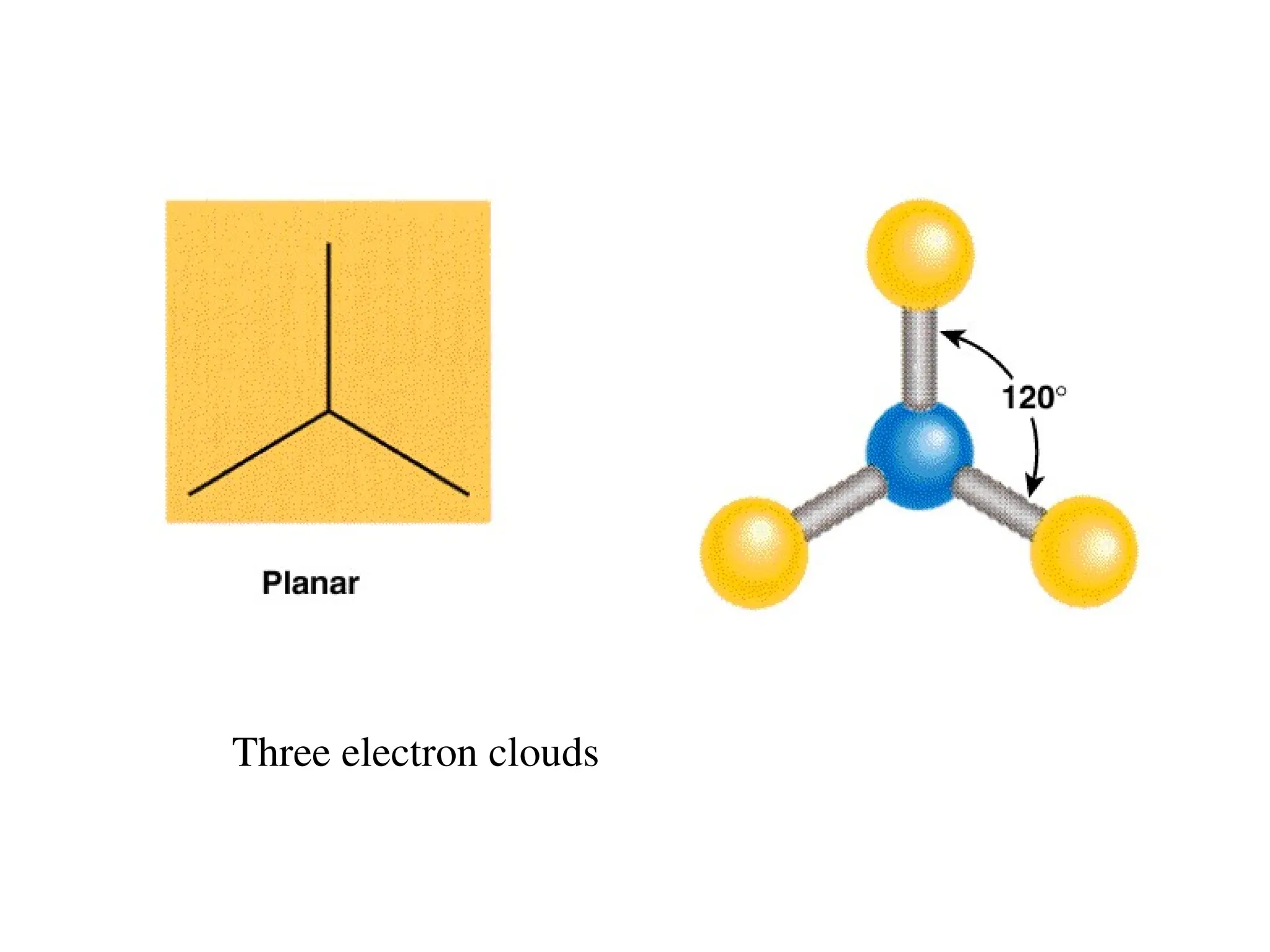
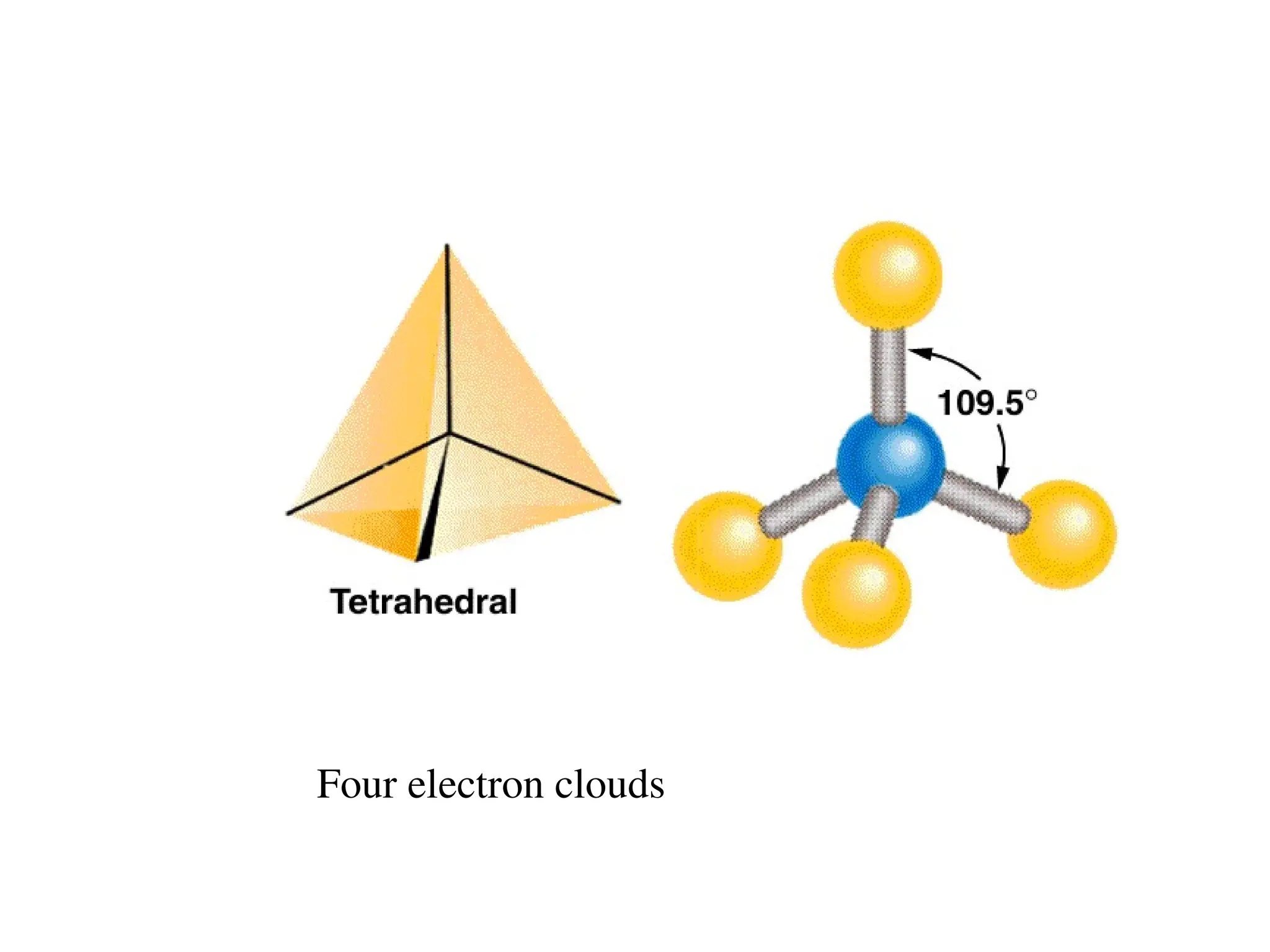
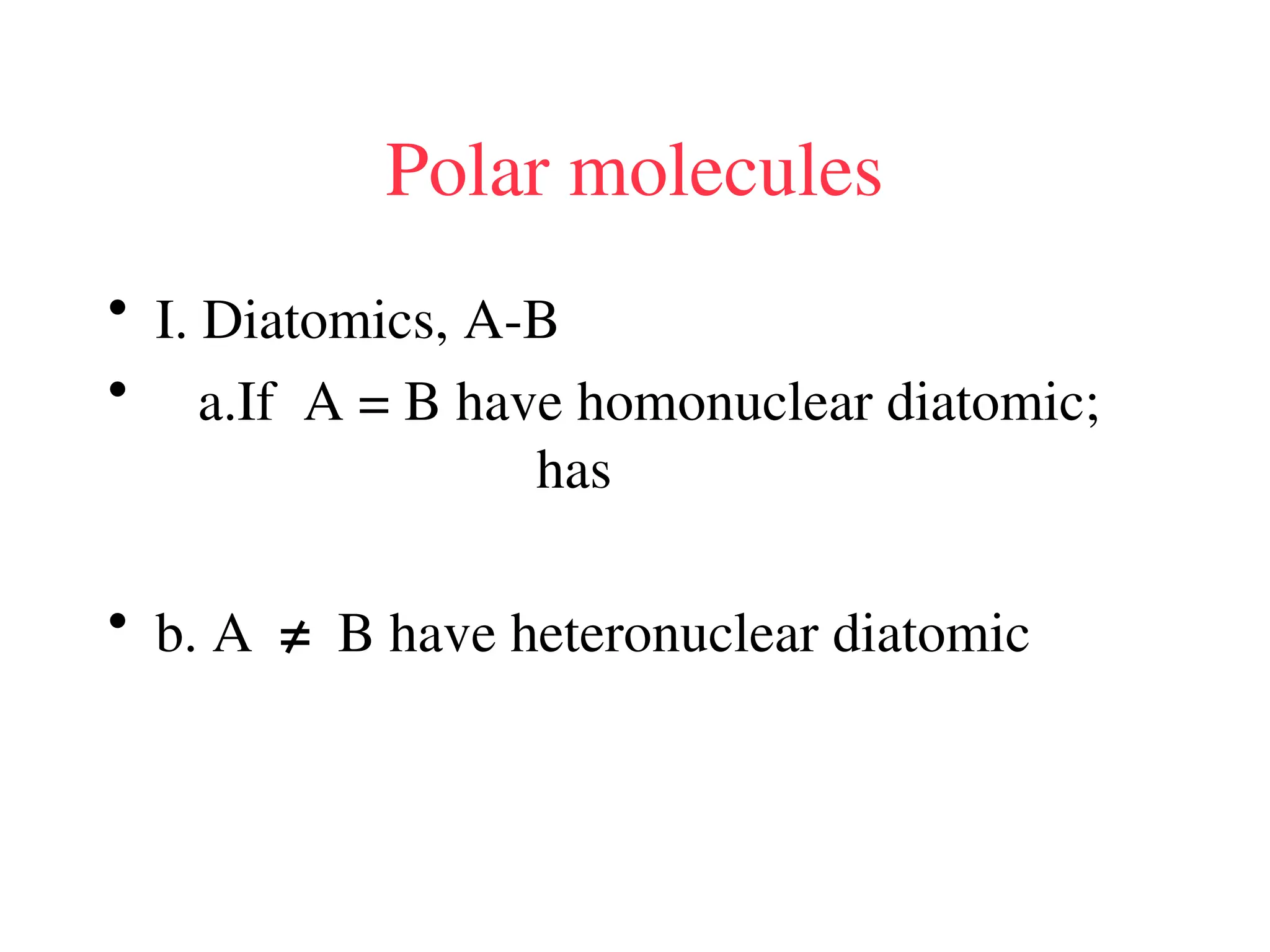
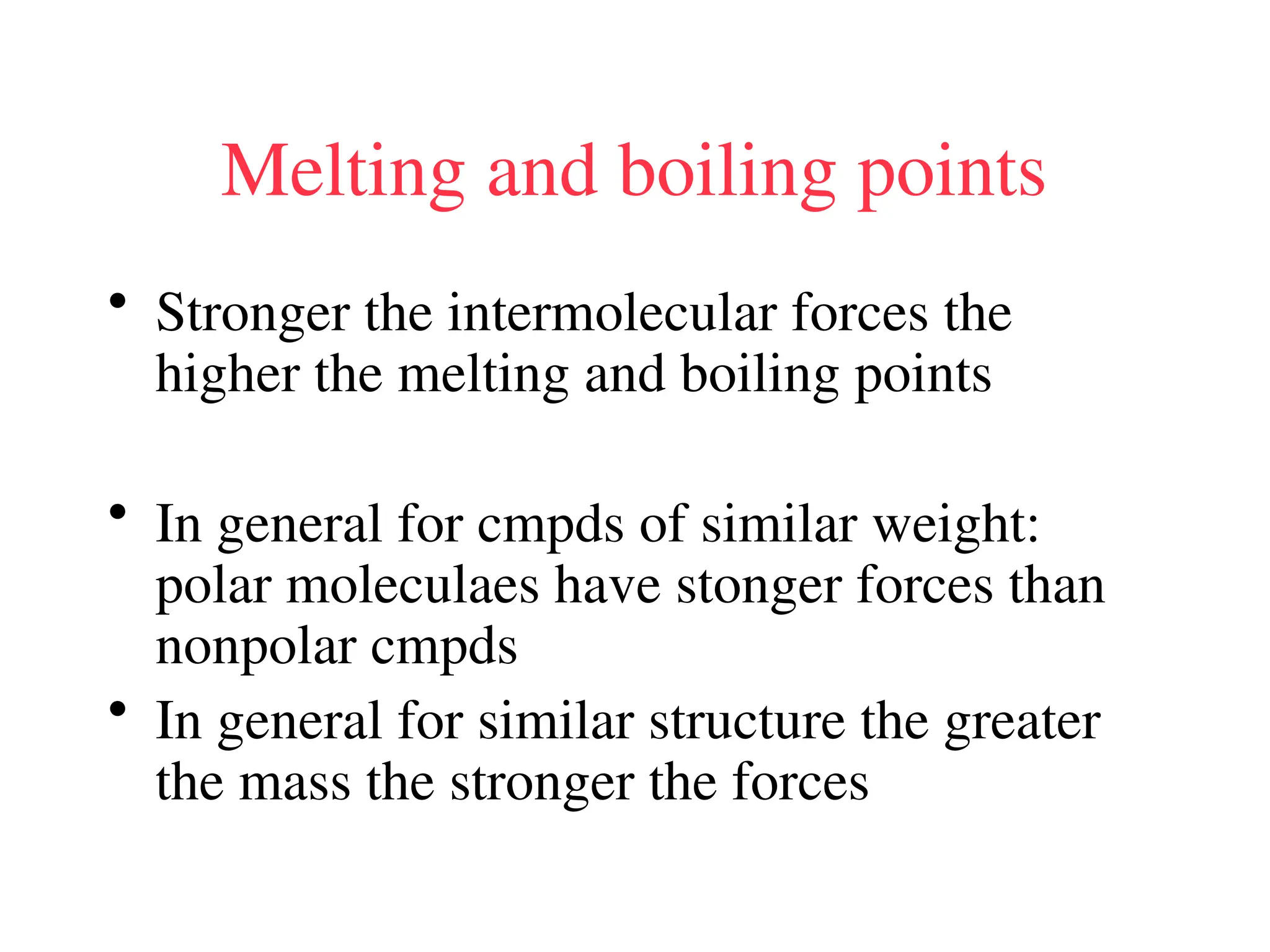
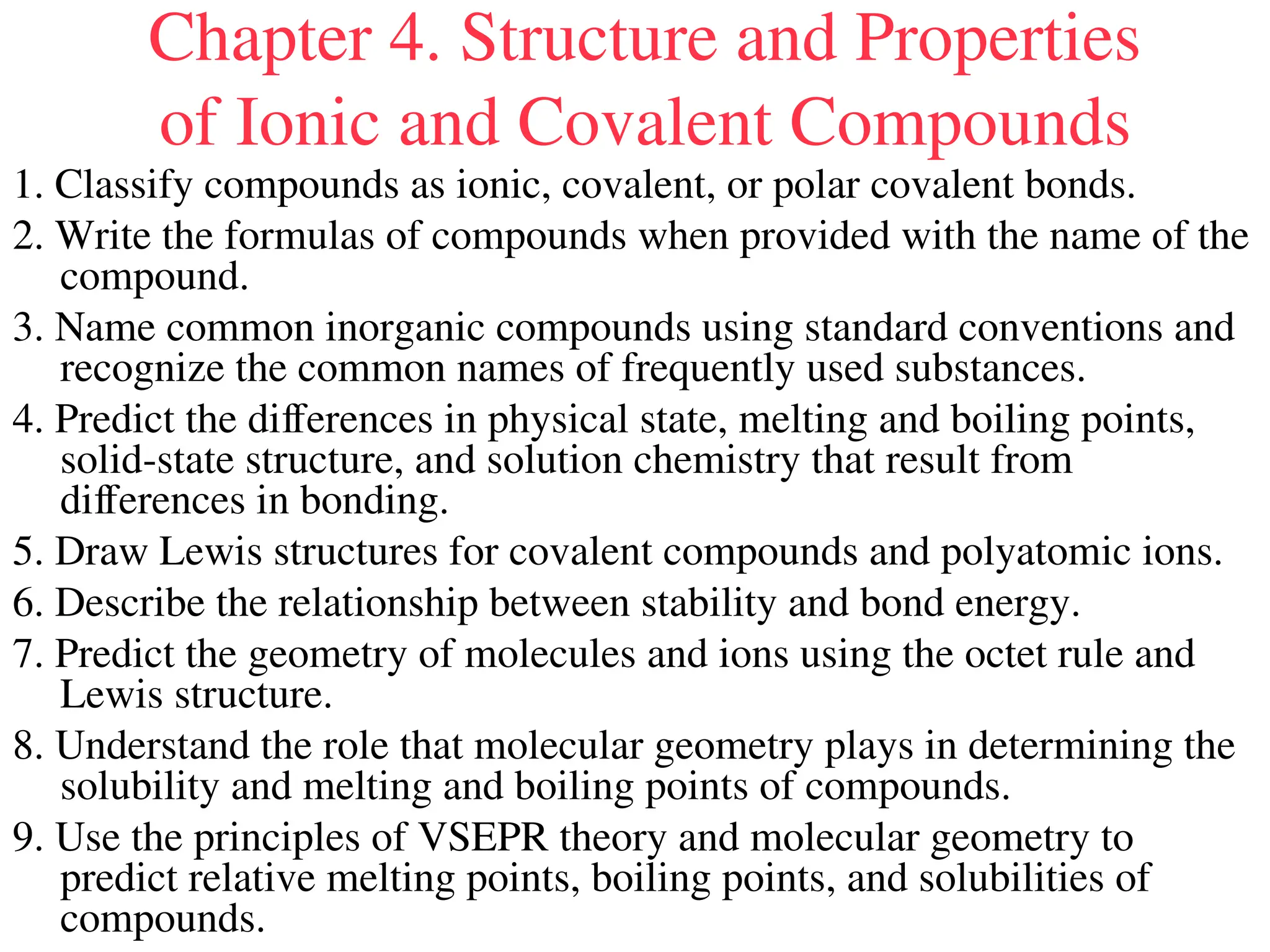
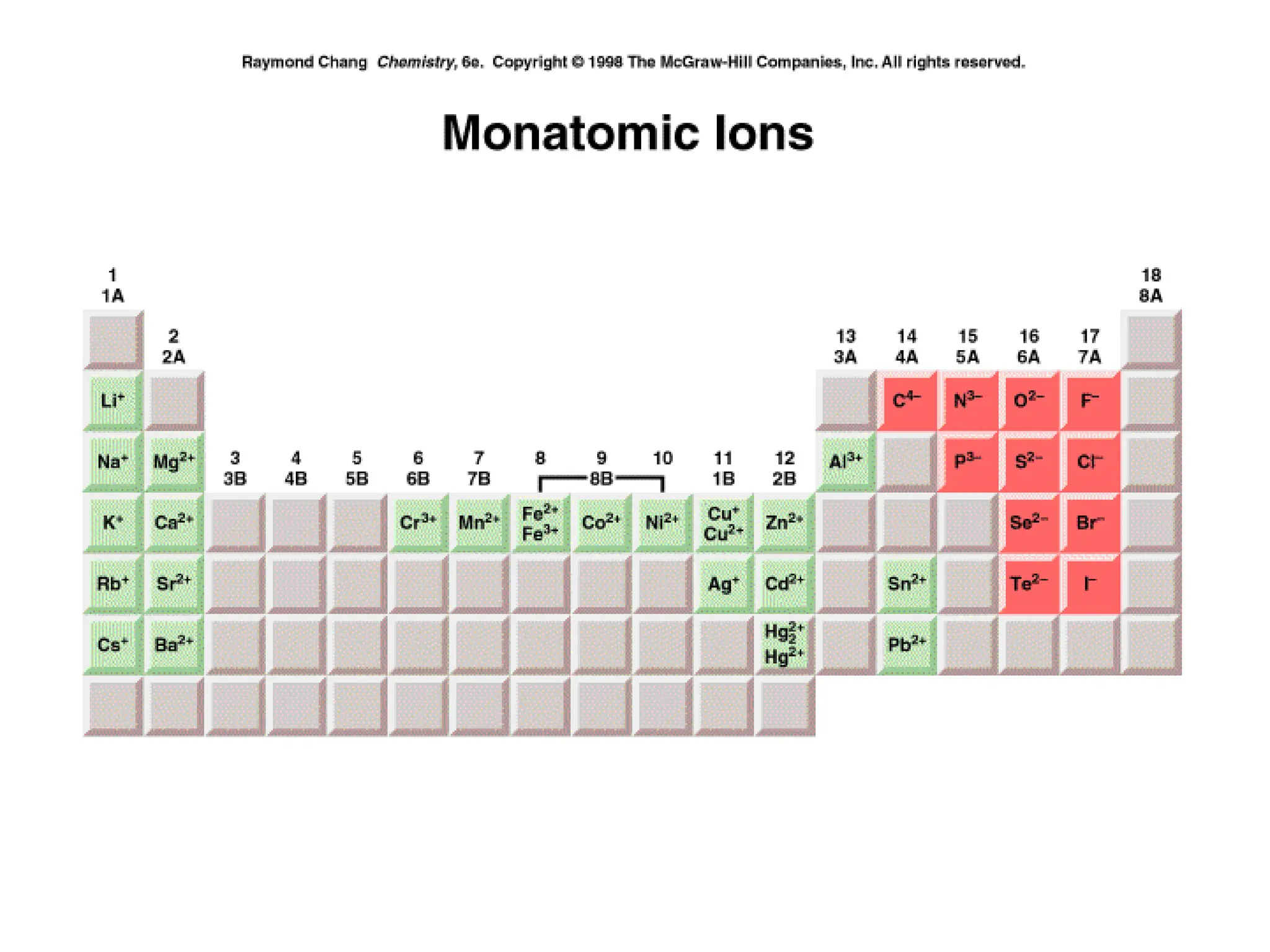
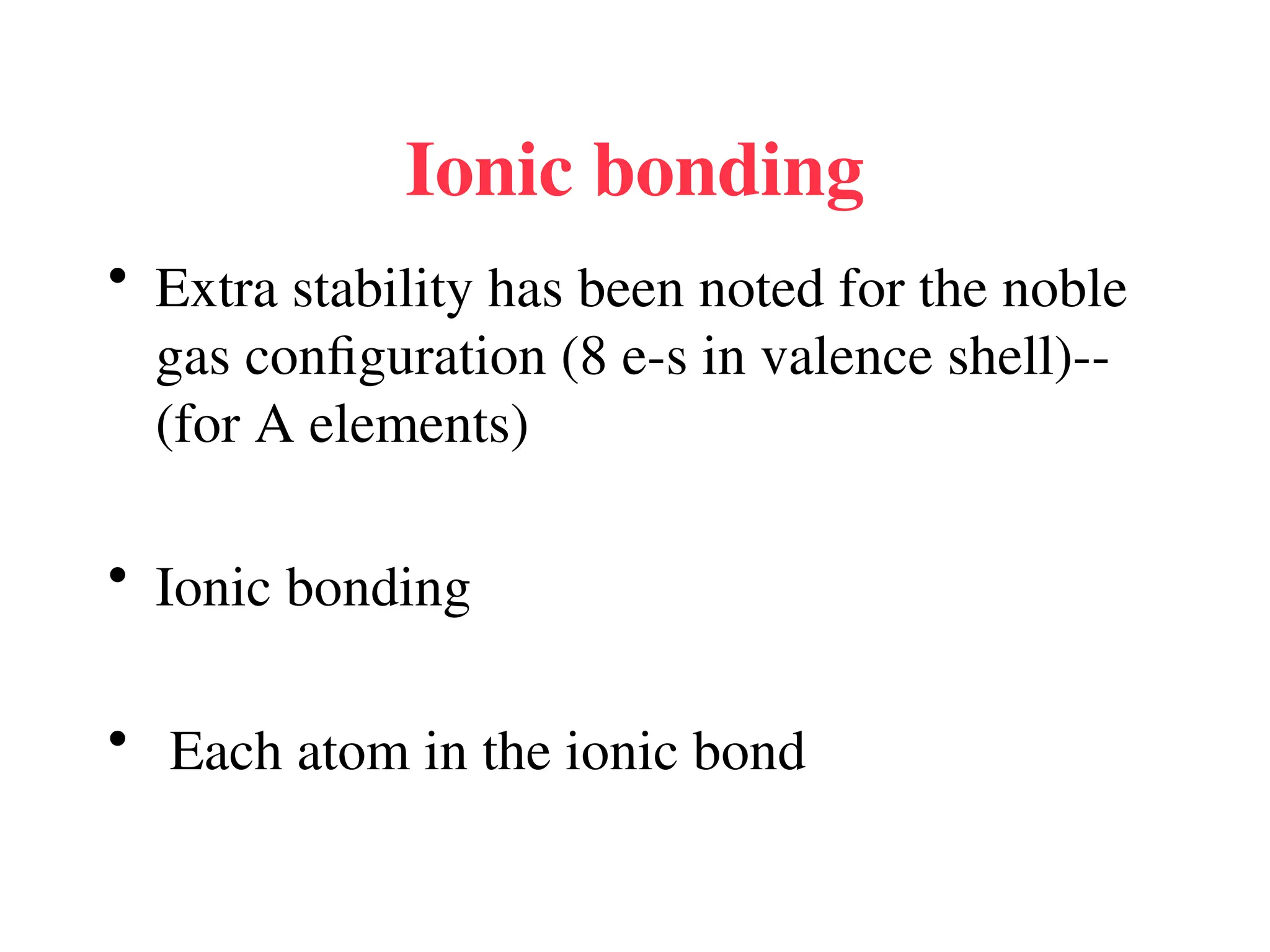
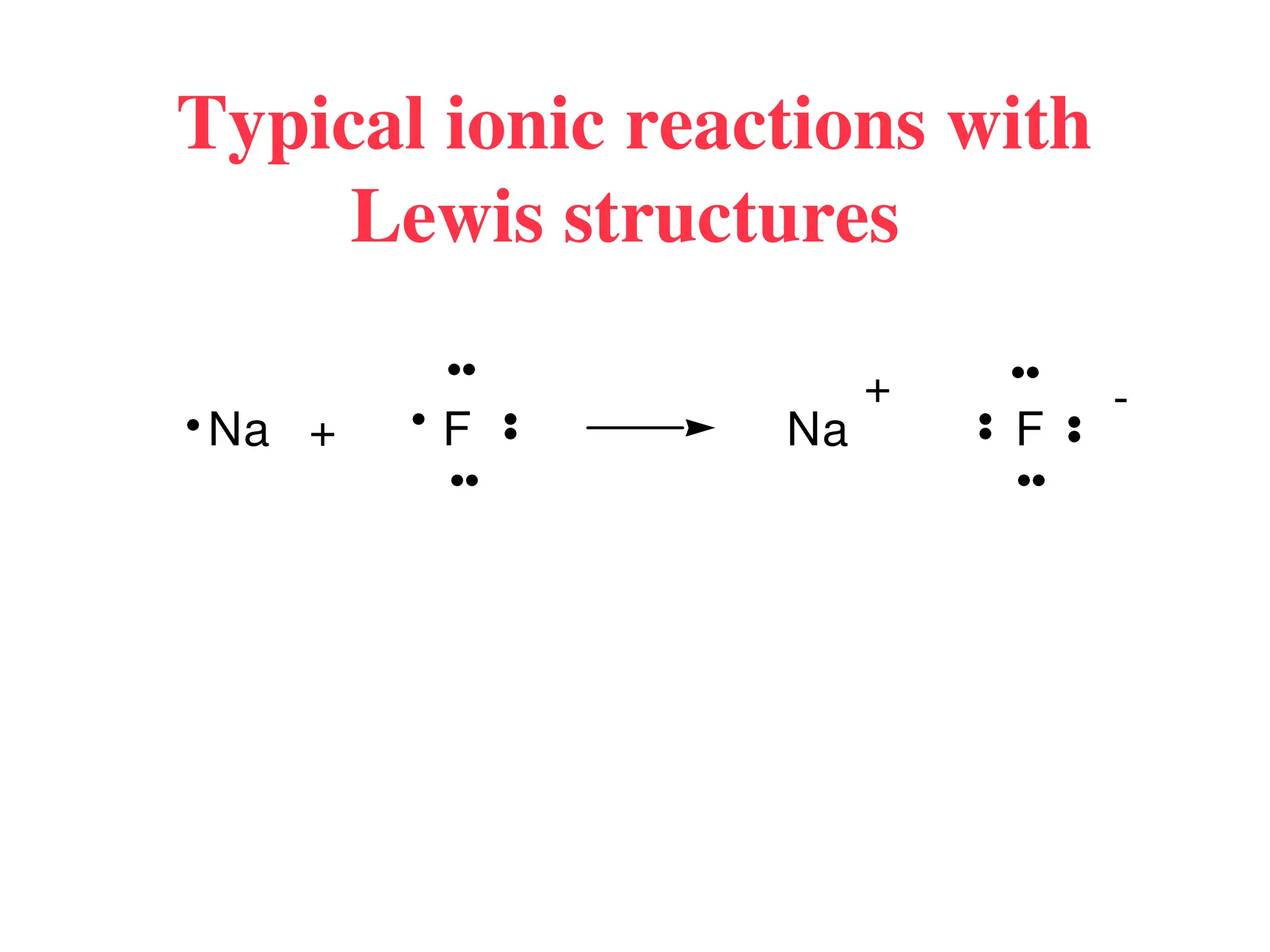
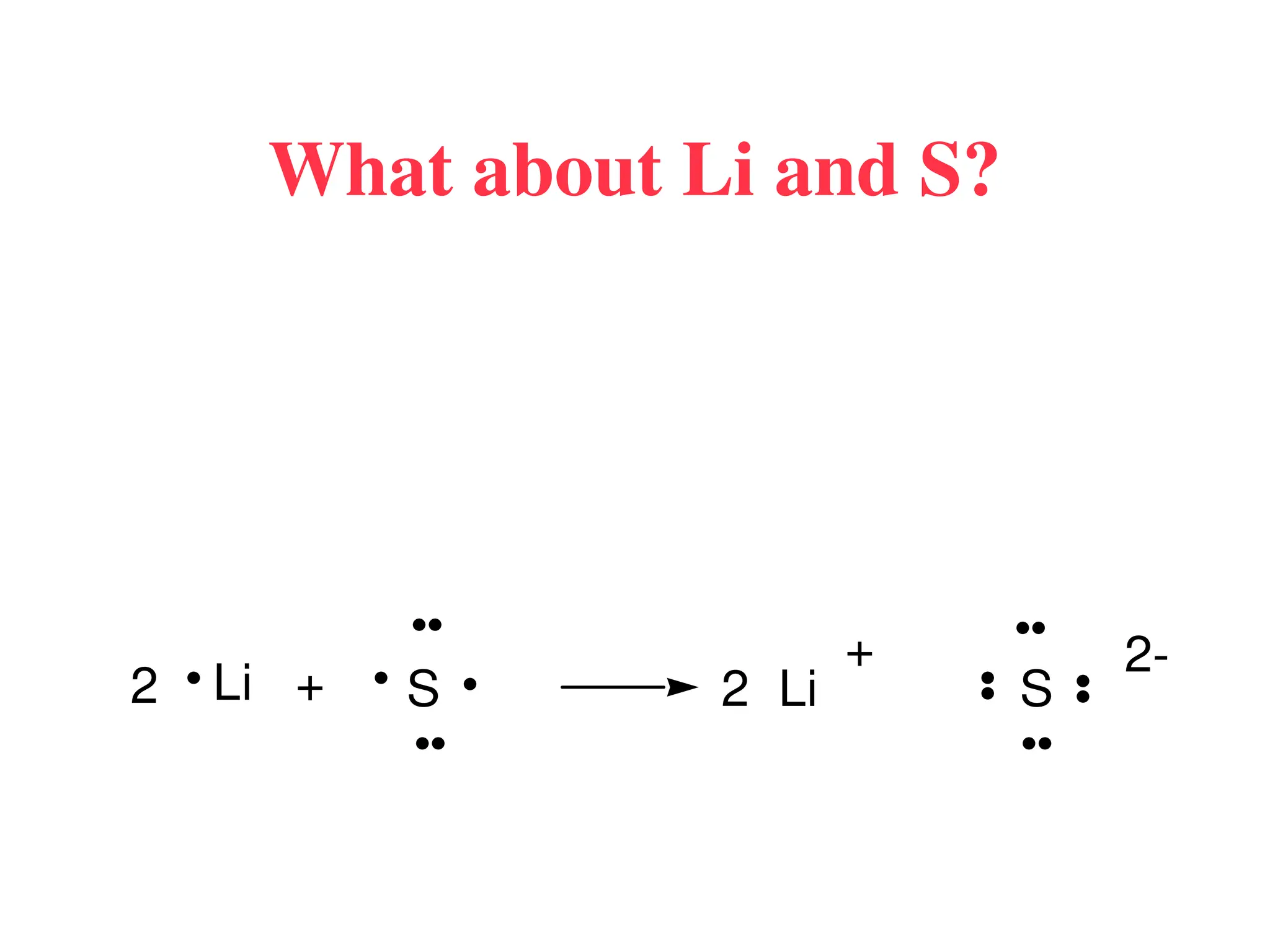
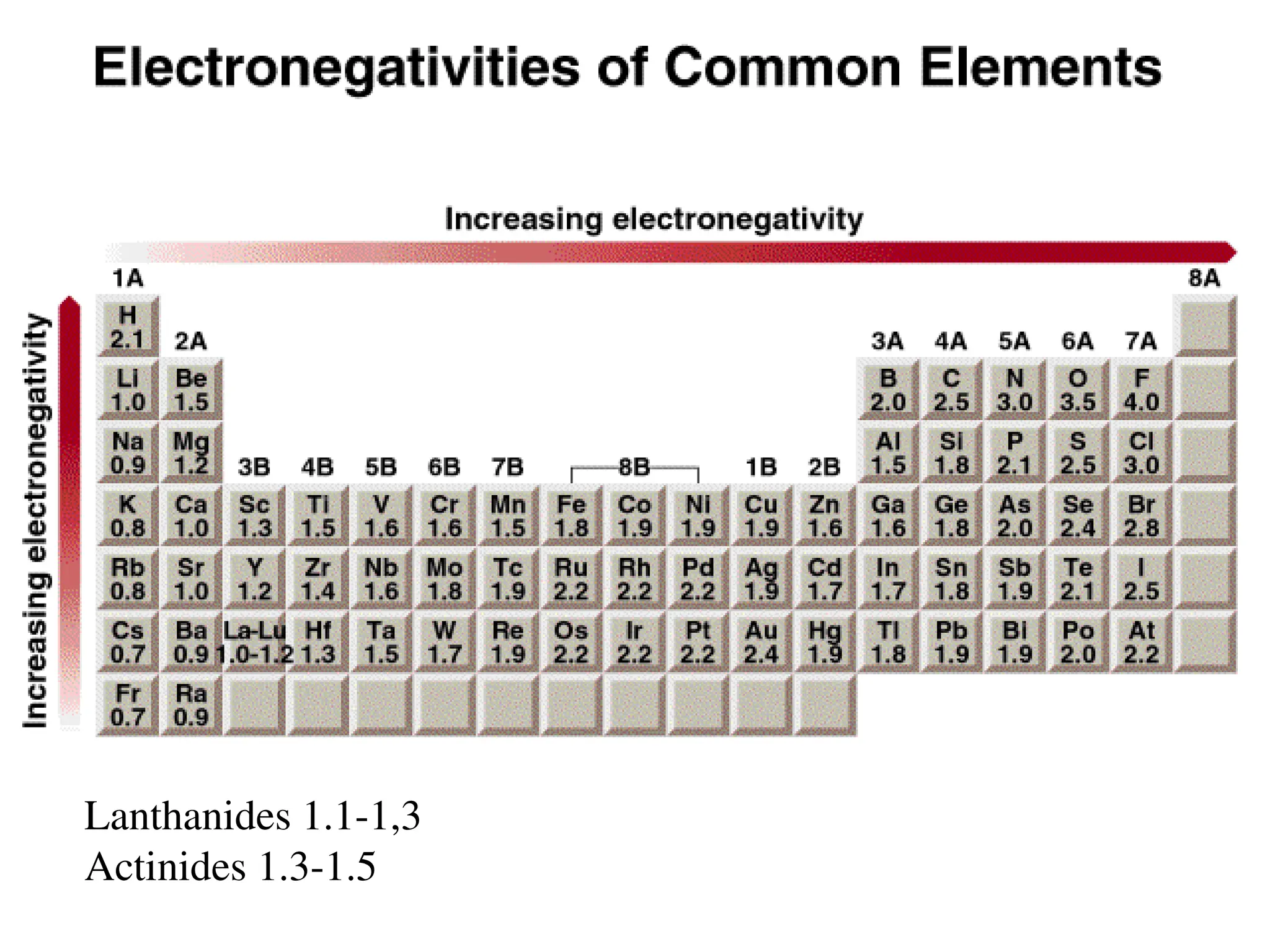
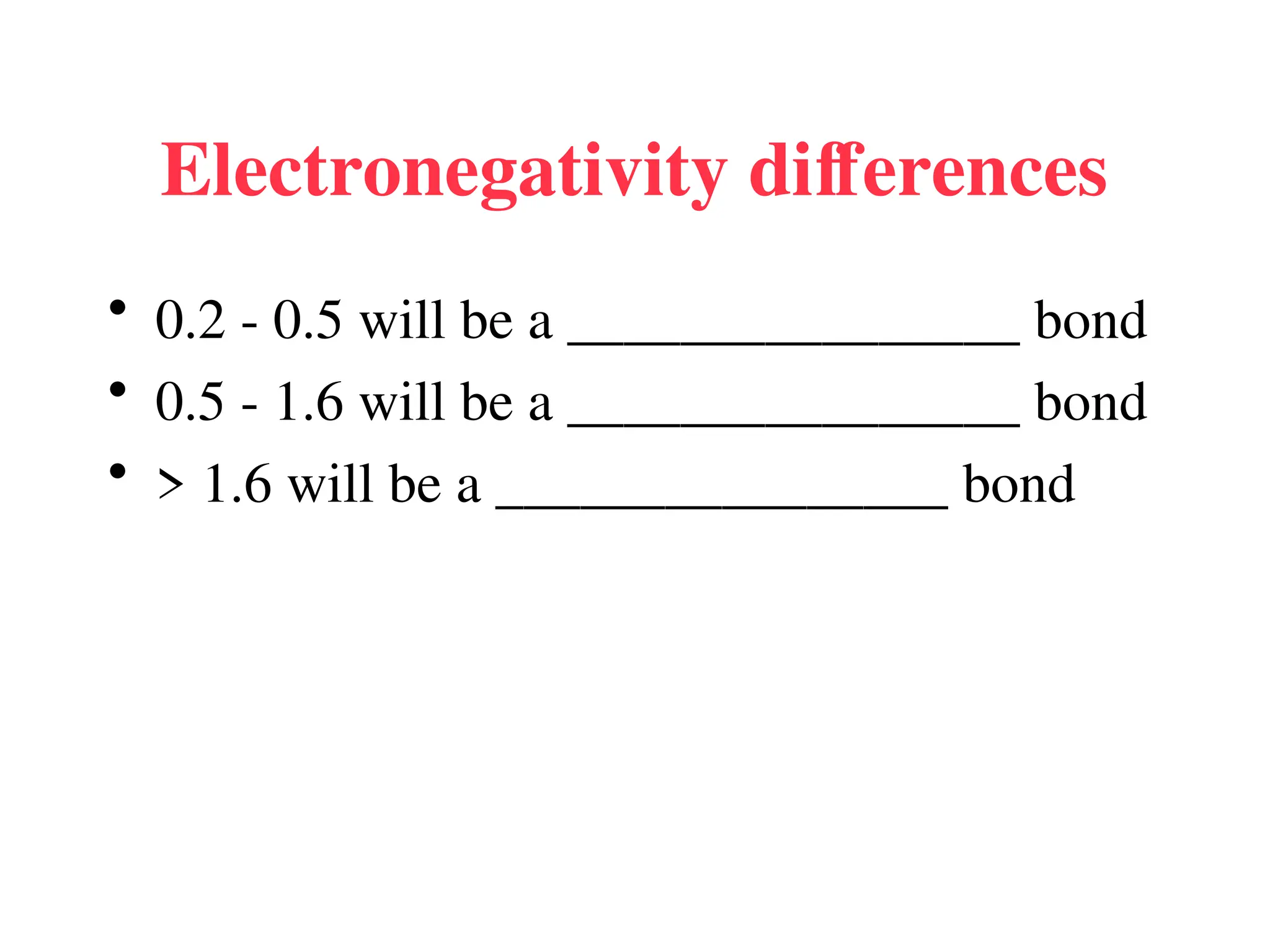
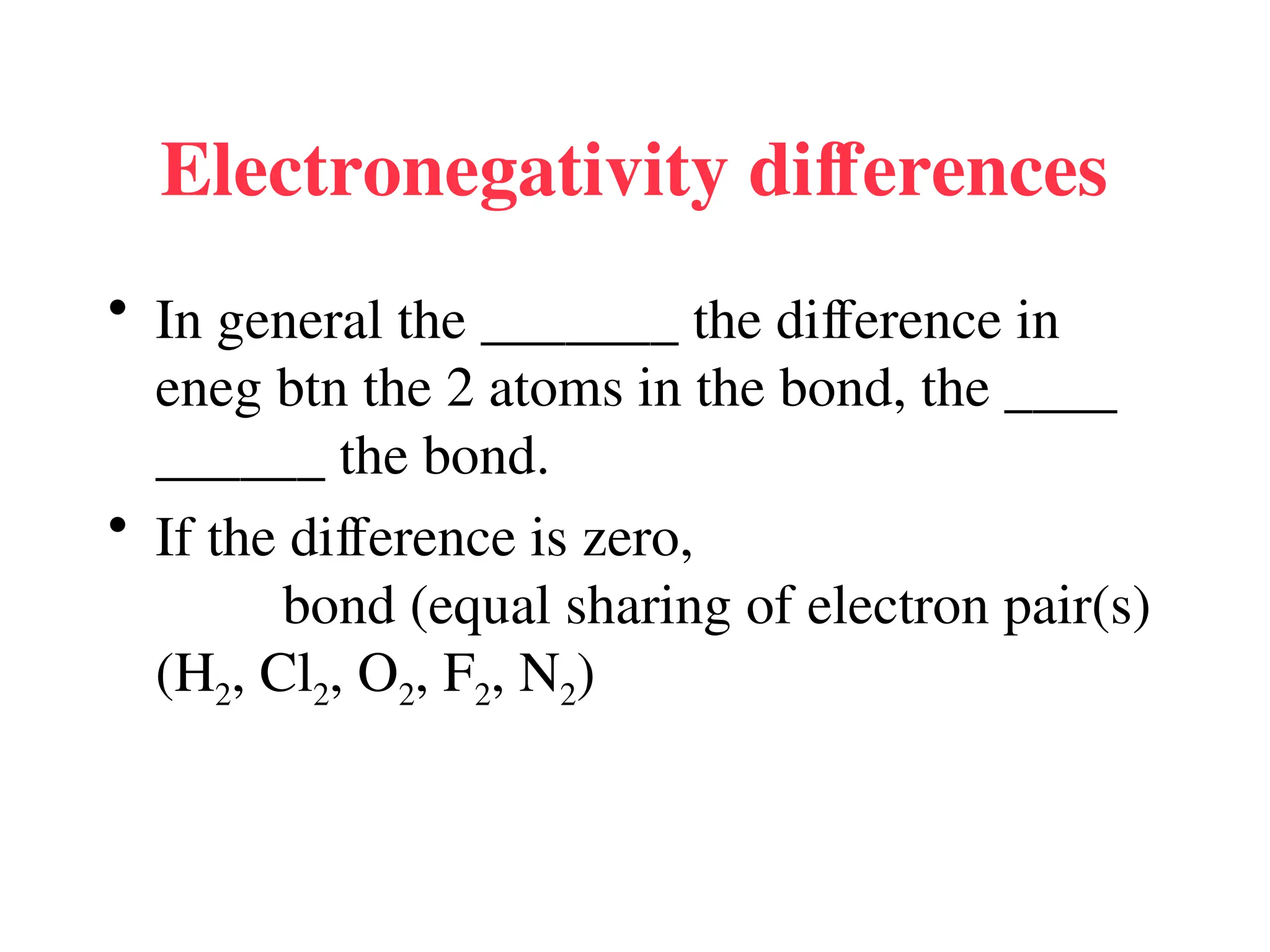
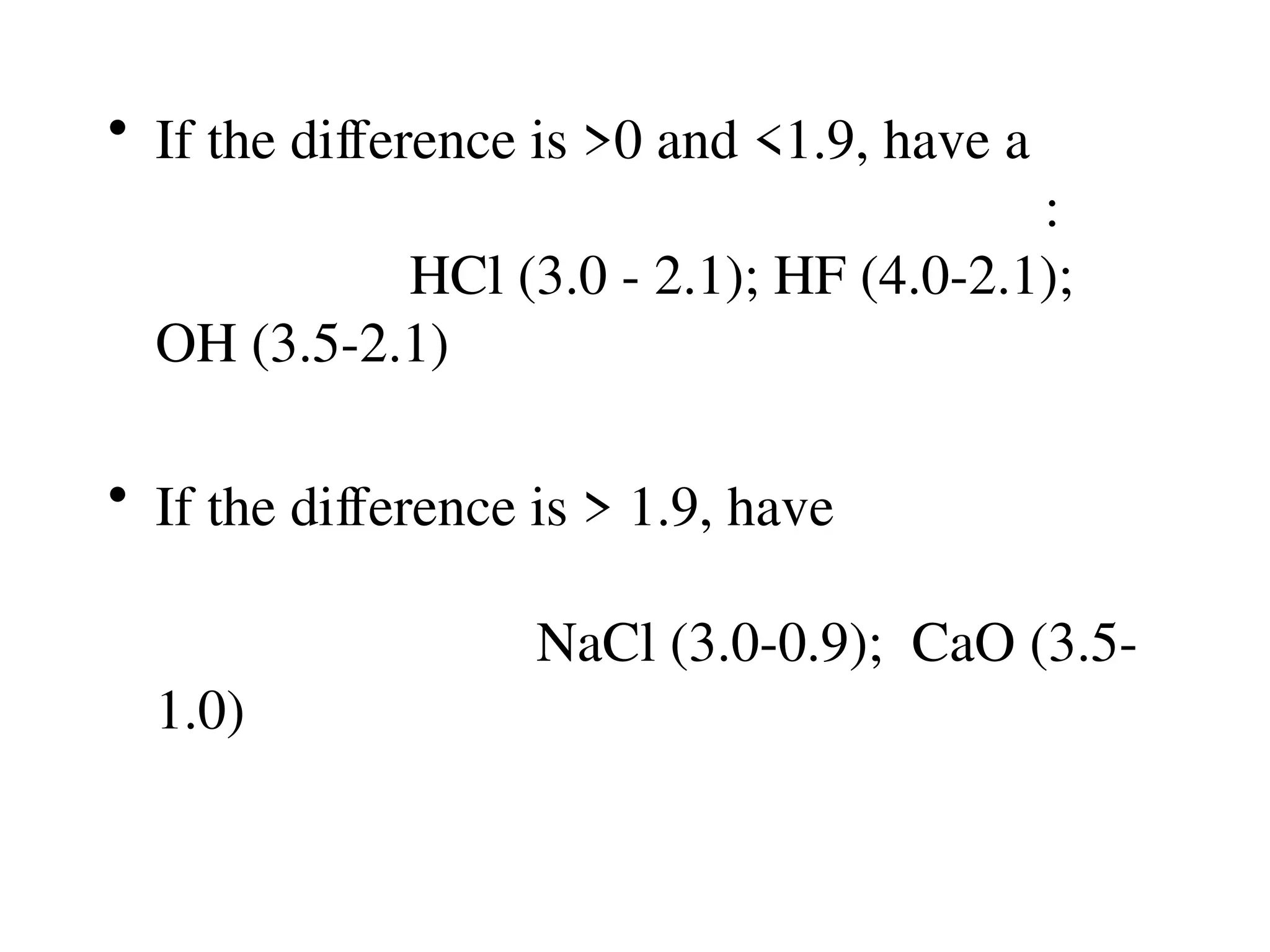
The document is a syllabus for an introductory inorganic chemistry course (Chem 120) taught by Dr. Upali Siriwardane, detailing course structure, test dates, grading criteria, and topics covered, including ionic and covalent bonds, molecular geometry, and naming conventions for compounds. It outlines the learning objectives for the course, such as classifying compounds, predicting properties based on bonding, and writing chemical formulas. The document emphasizes the importance of understanding valence electrons, bond types, and the properties of molecular and ionic compounds.

![Chapters Covered and Test dates
Chapters Covered and Test dates
• Tests will be given in regular class periods from 9:30-10:45 a.m. on
the following days:
September 22, 2004 (Test 1): Chapters 1 & 2
• October 6, 2004(Test 2): Chapters 3, & 4
• October 20, 2004 (Test 3): Chapter 5 & 6
• November 3, 2004 (Test 4): Chapter 7 & 8
• November 15, 2004 (Test 5): Chapter 9 & 10
• November 17, 2004 MAKE-UP: Comprehensive test (Covers all
chapters
• Grading:
• [( Test 1 + Test 2 + Test3 + Test4 + Test5)] x.70 + [ Homework + quiz average] x 0.30 = Final Average
• 5](https://image.slidesharecdn.com/120ch04-240901093637-26e76639/75/Structure-and-properties-of-ionic-and-covalent-compounds-ppt-2-2048.jpg)



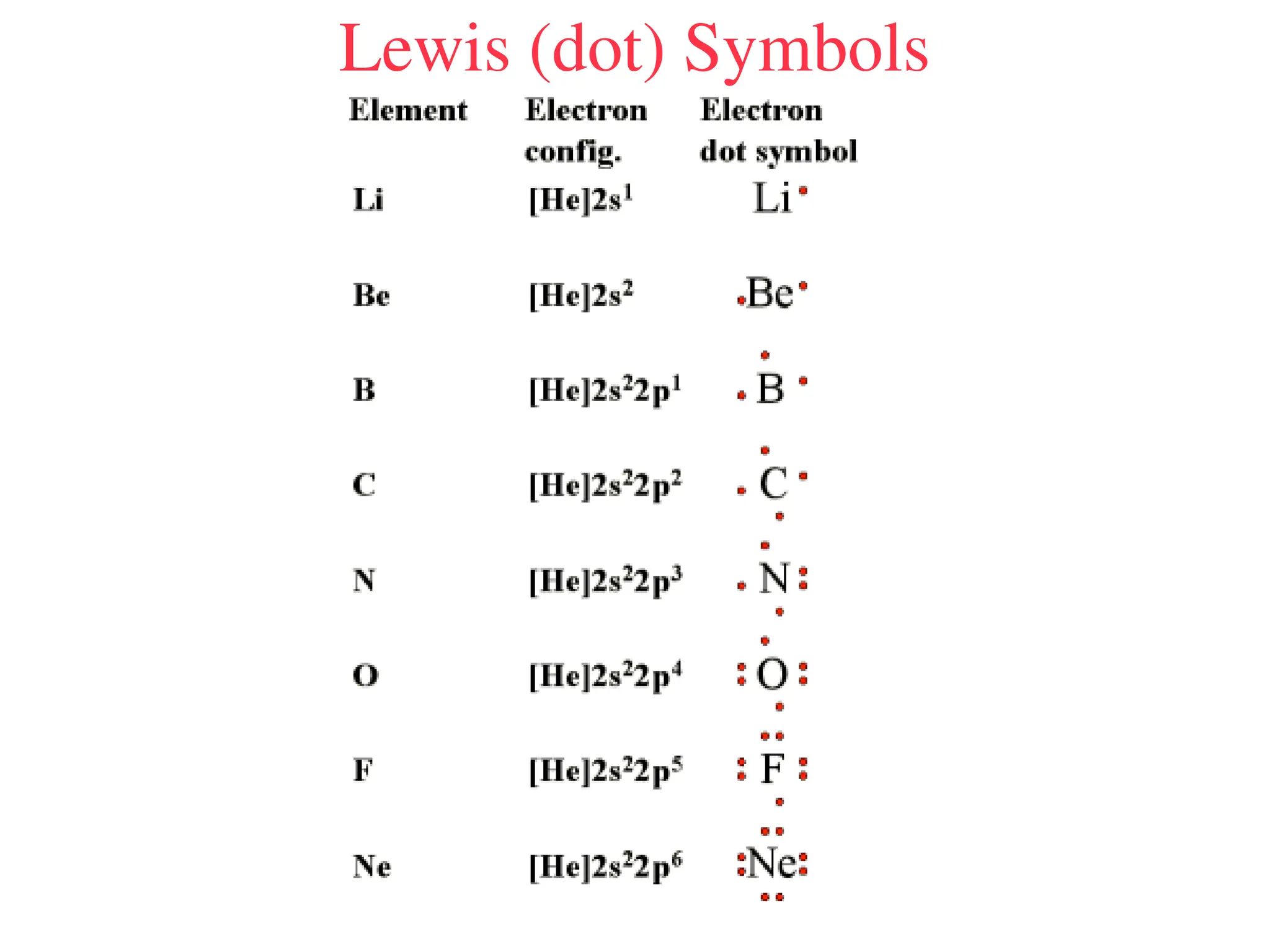















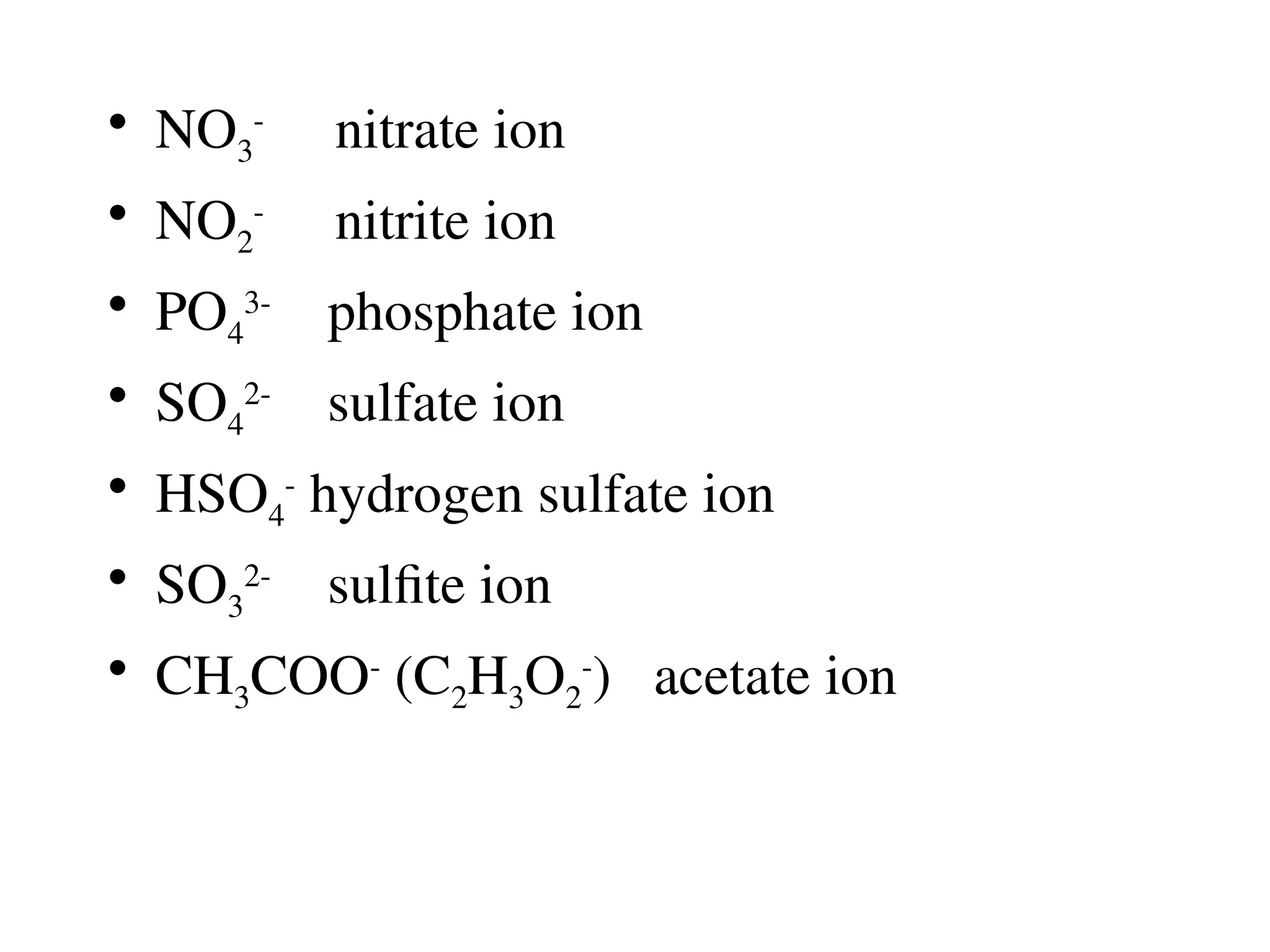









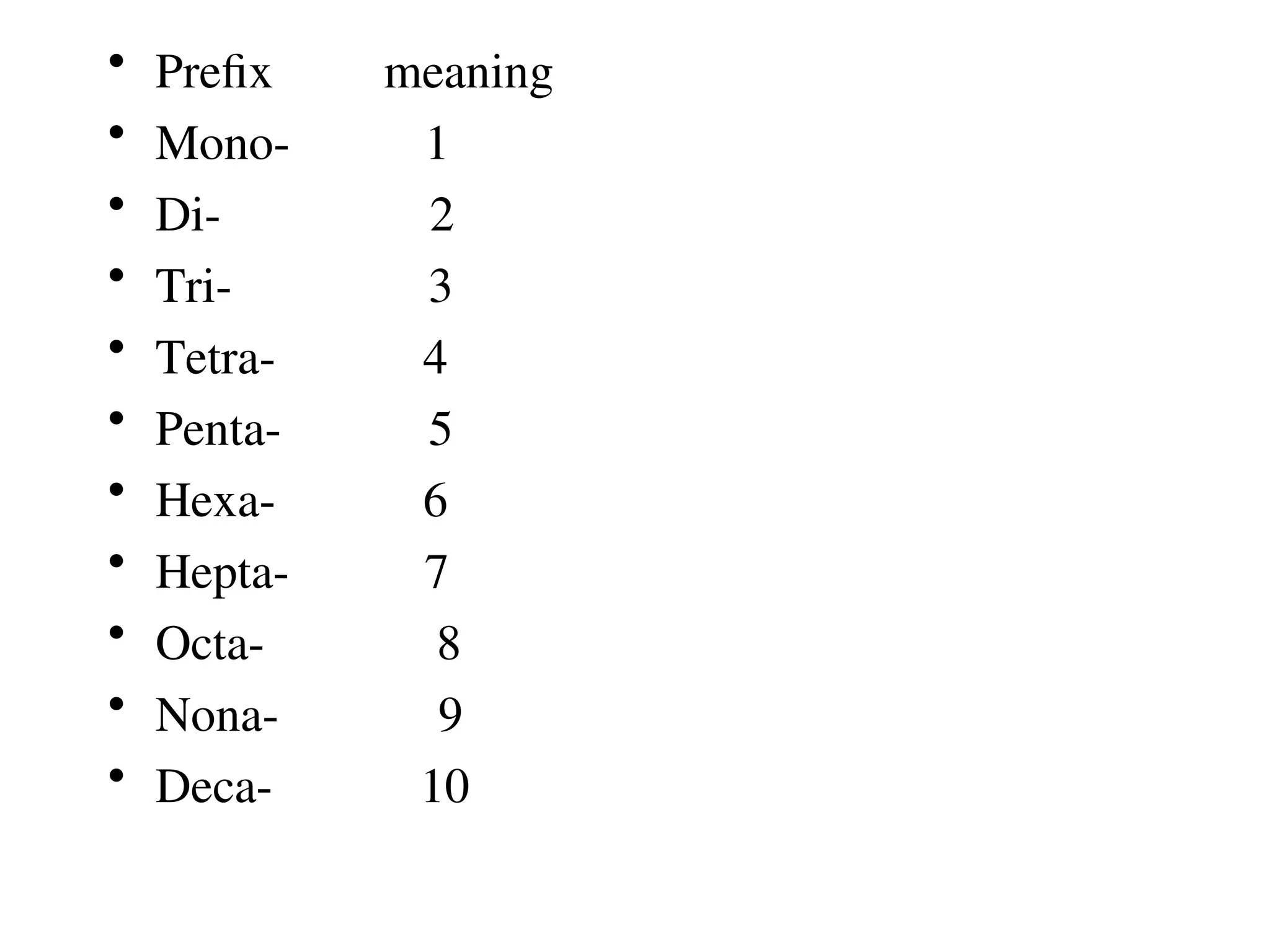
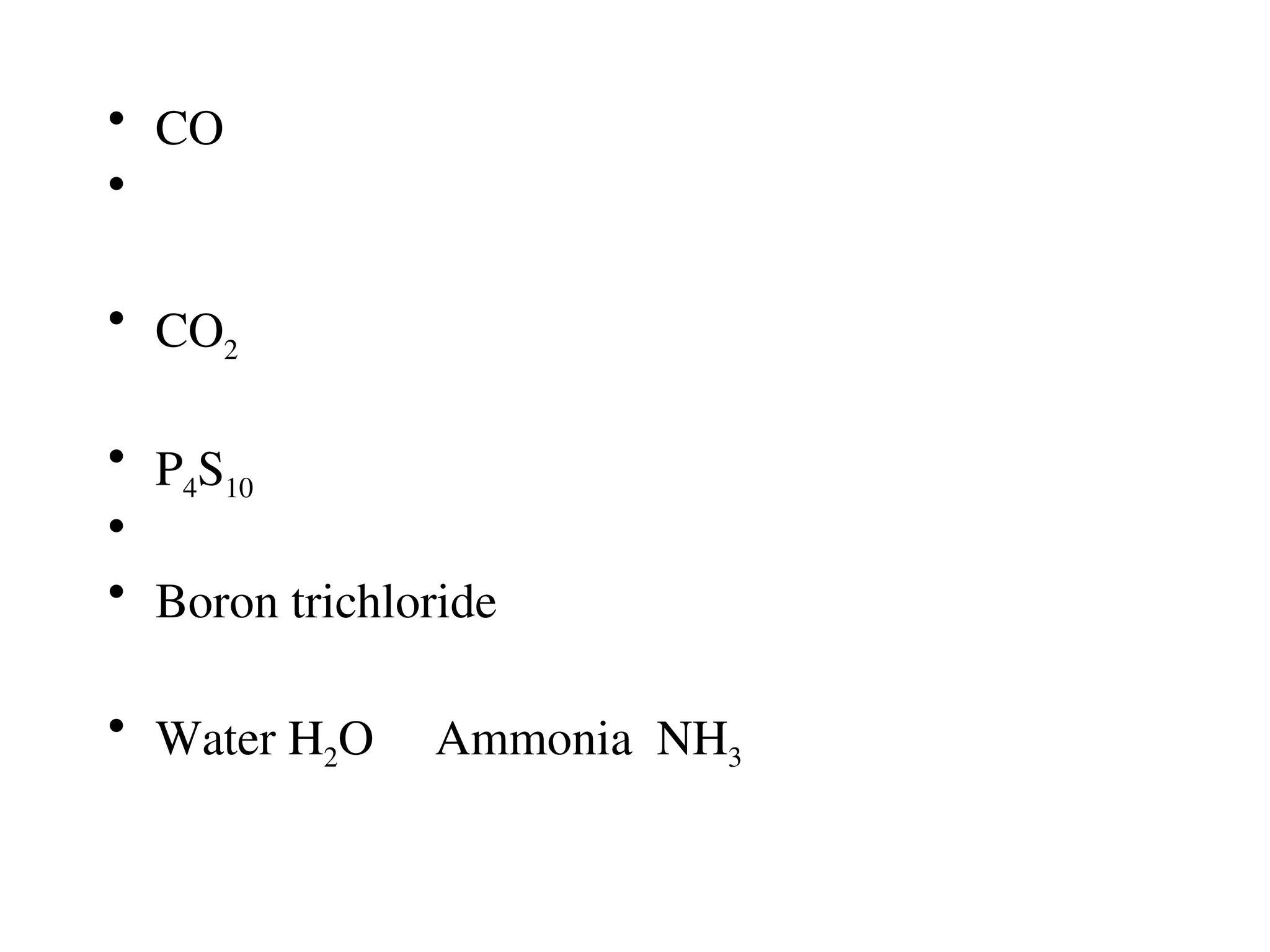

![Covalent cmpds
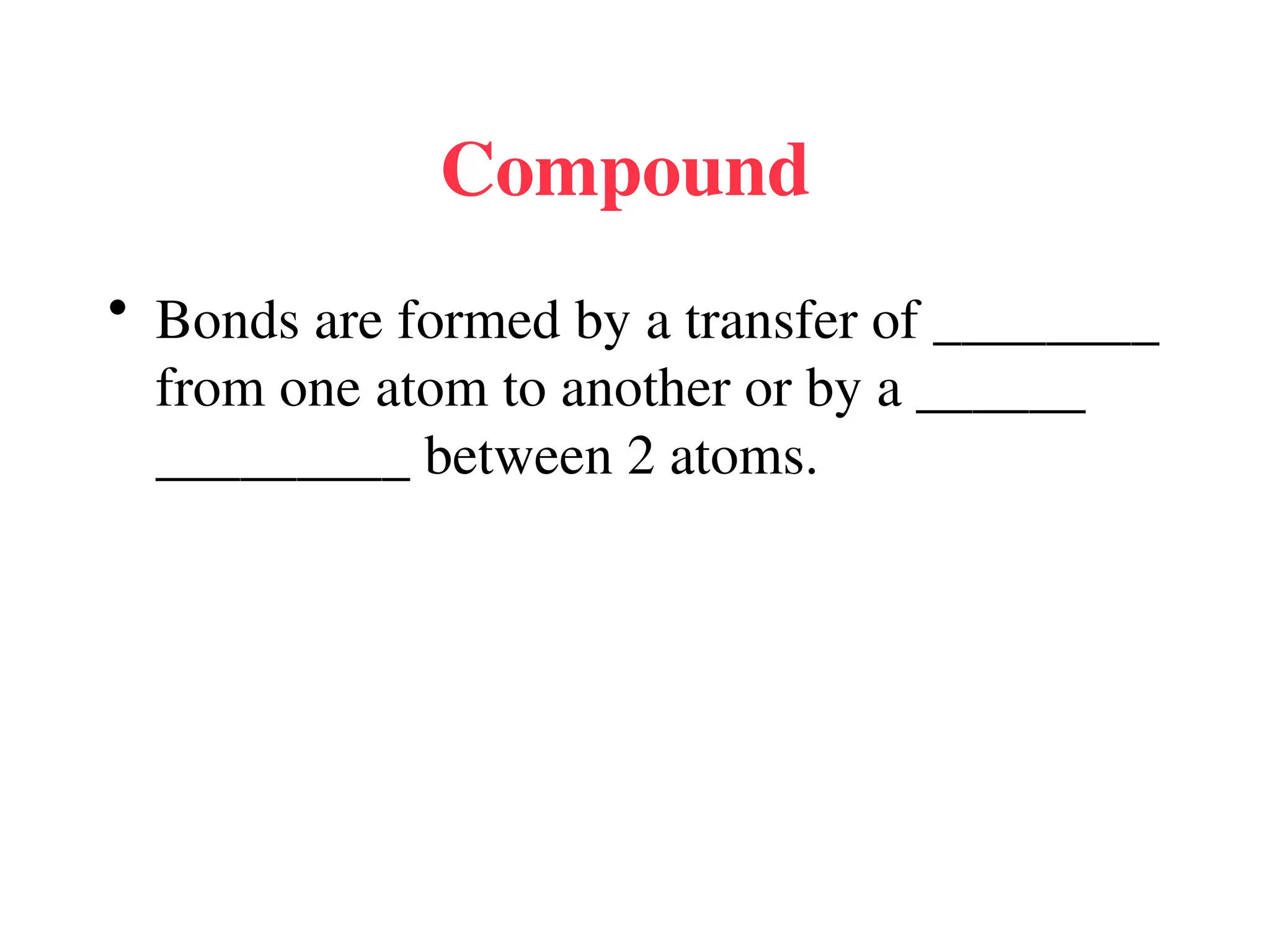
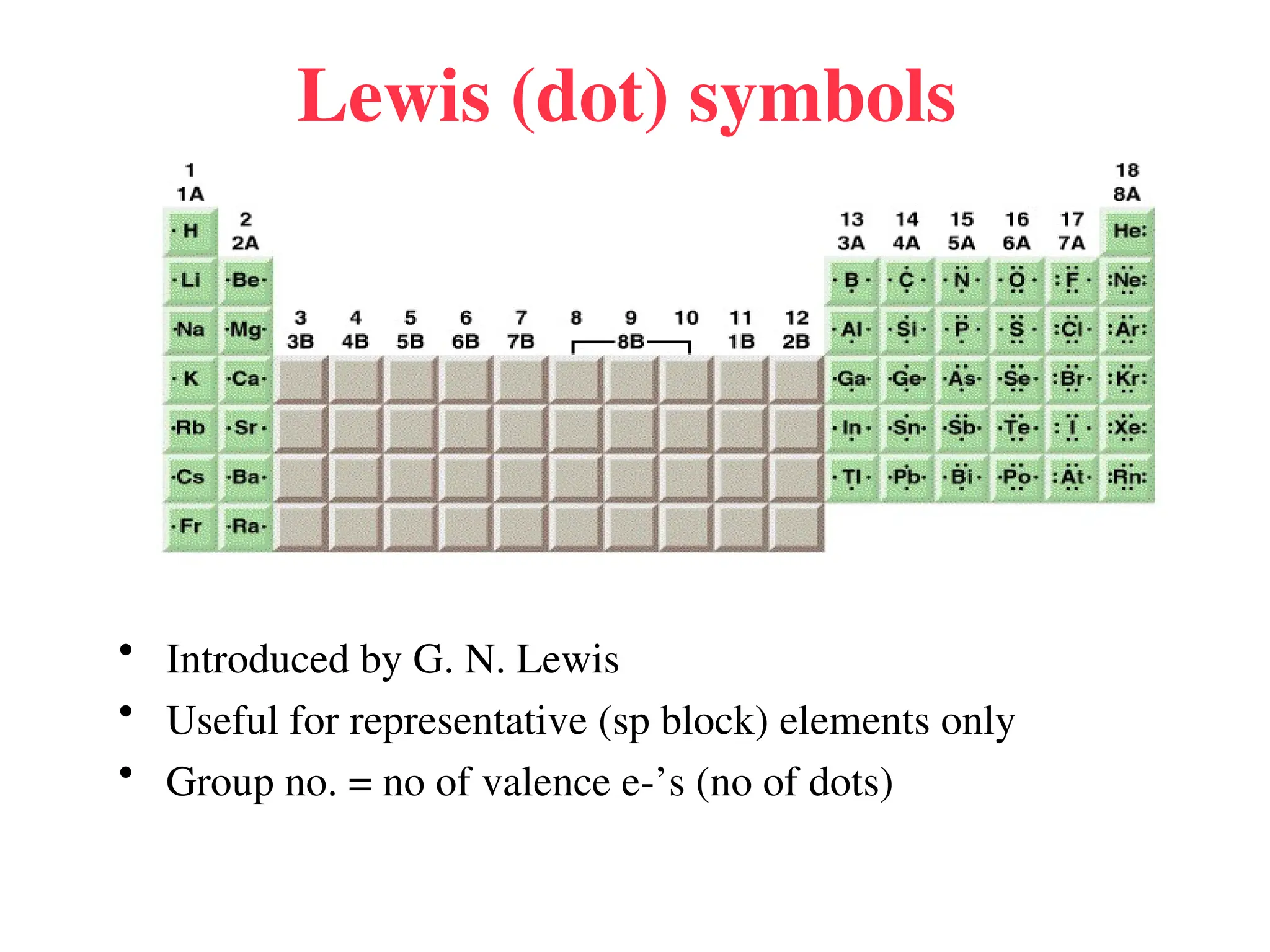
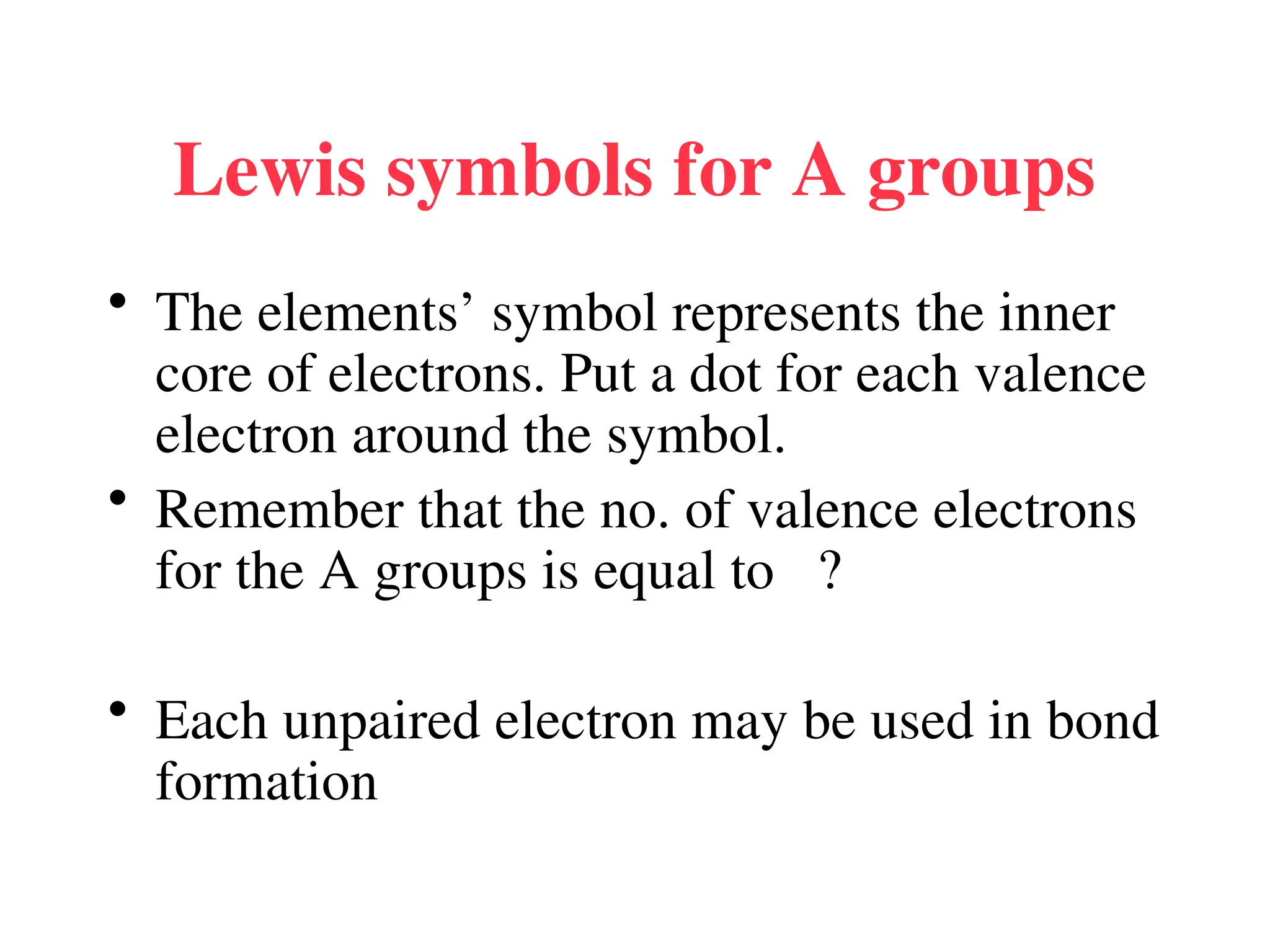
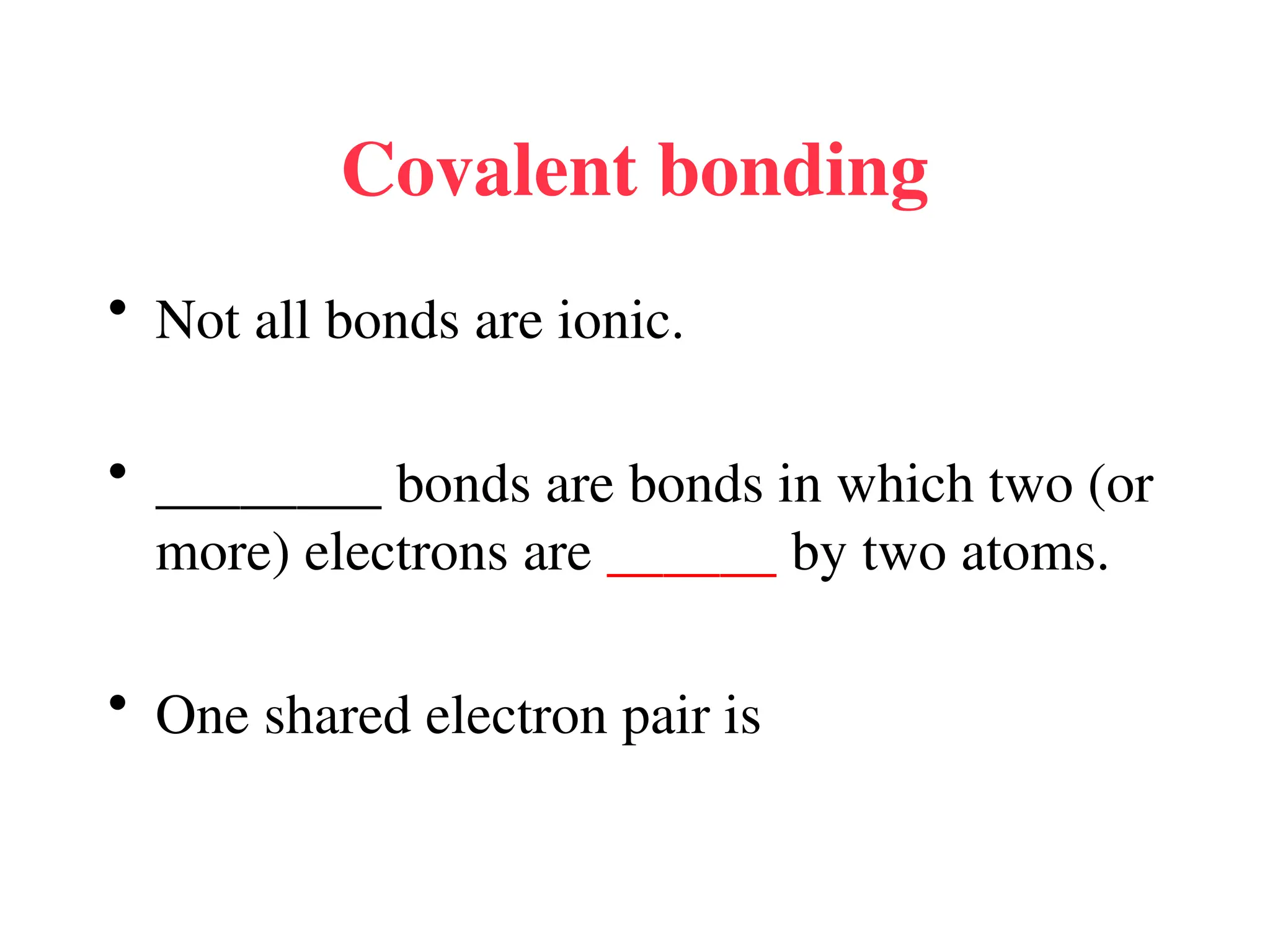
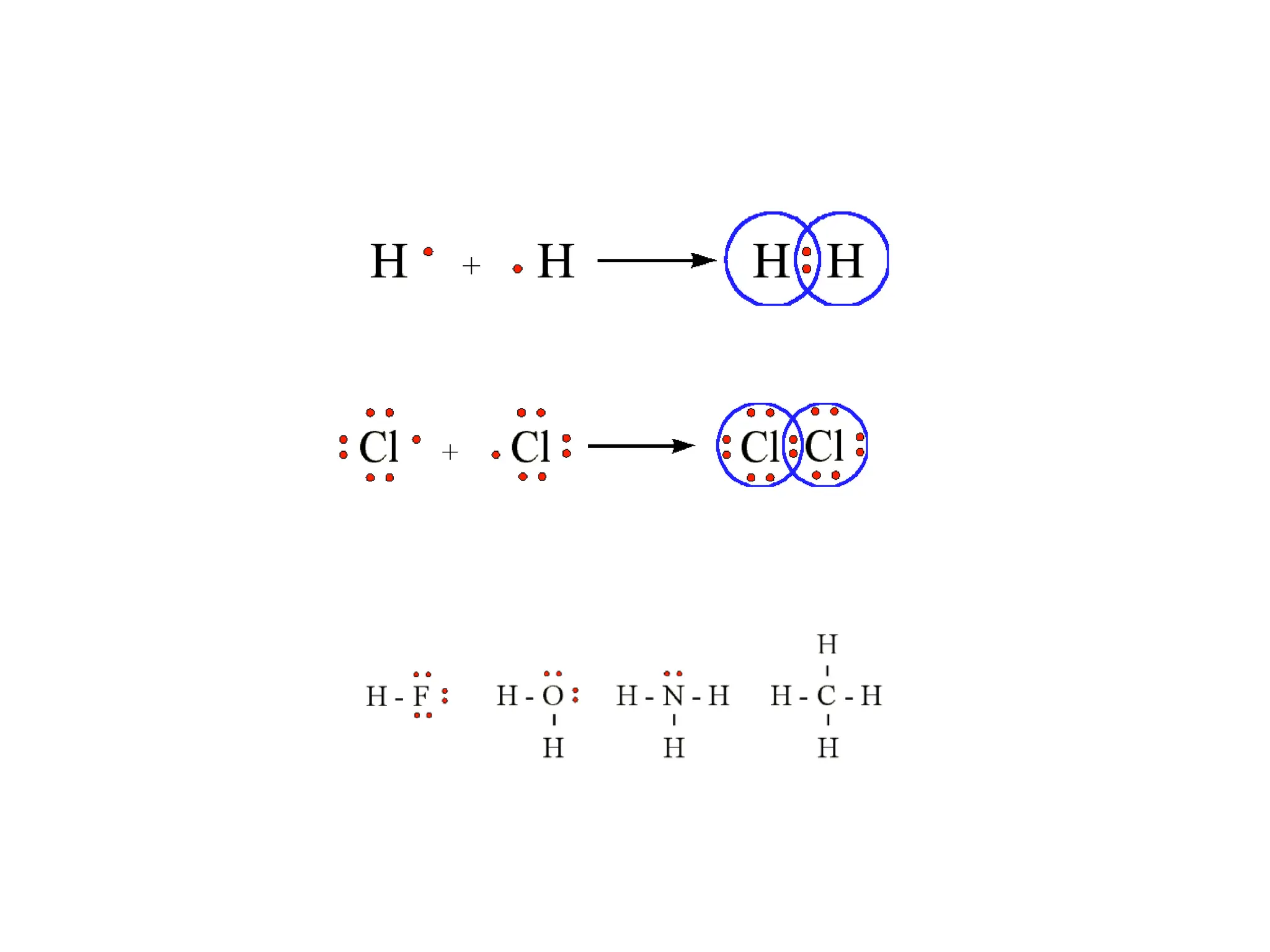
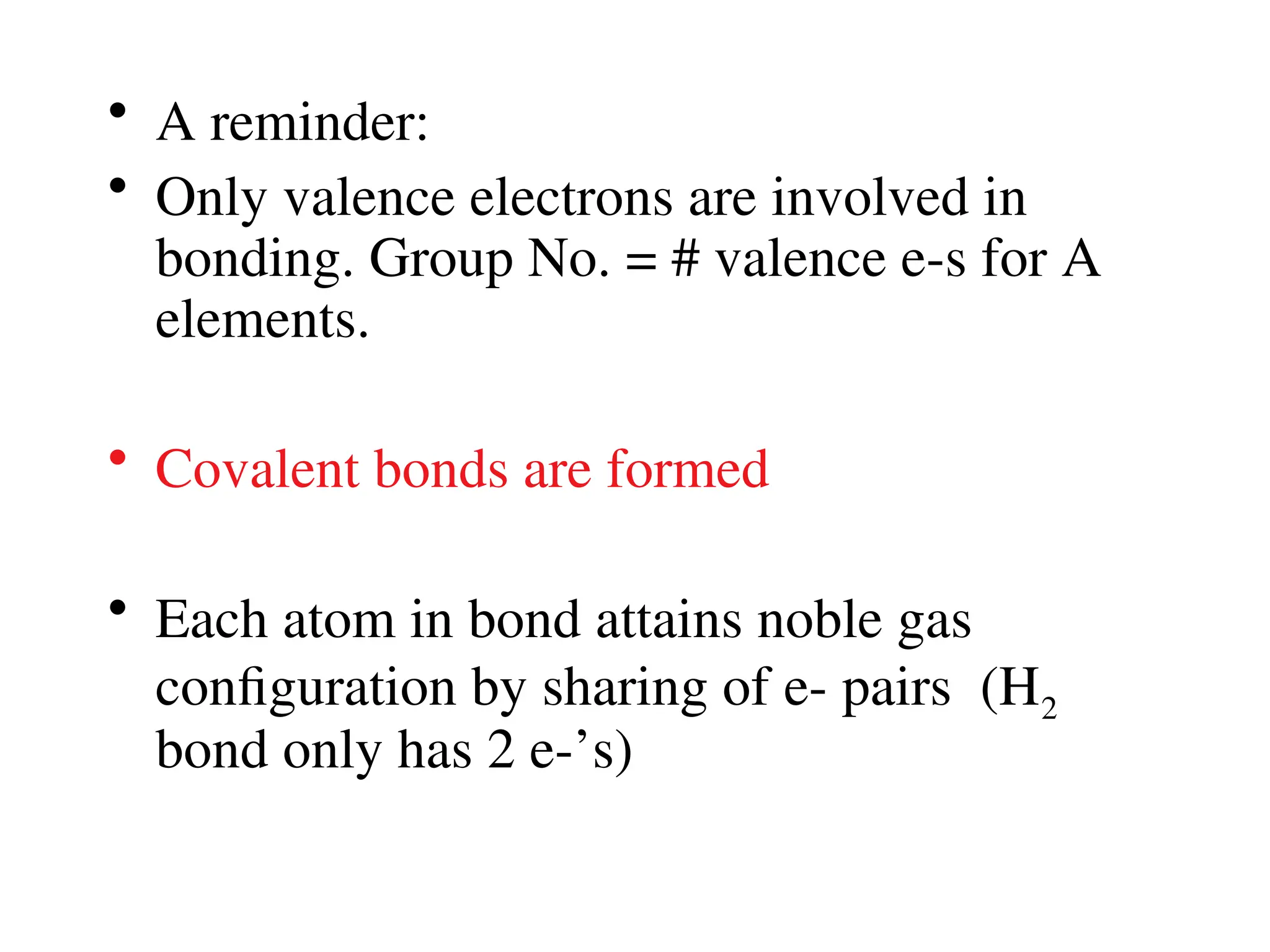
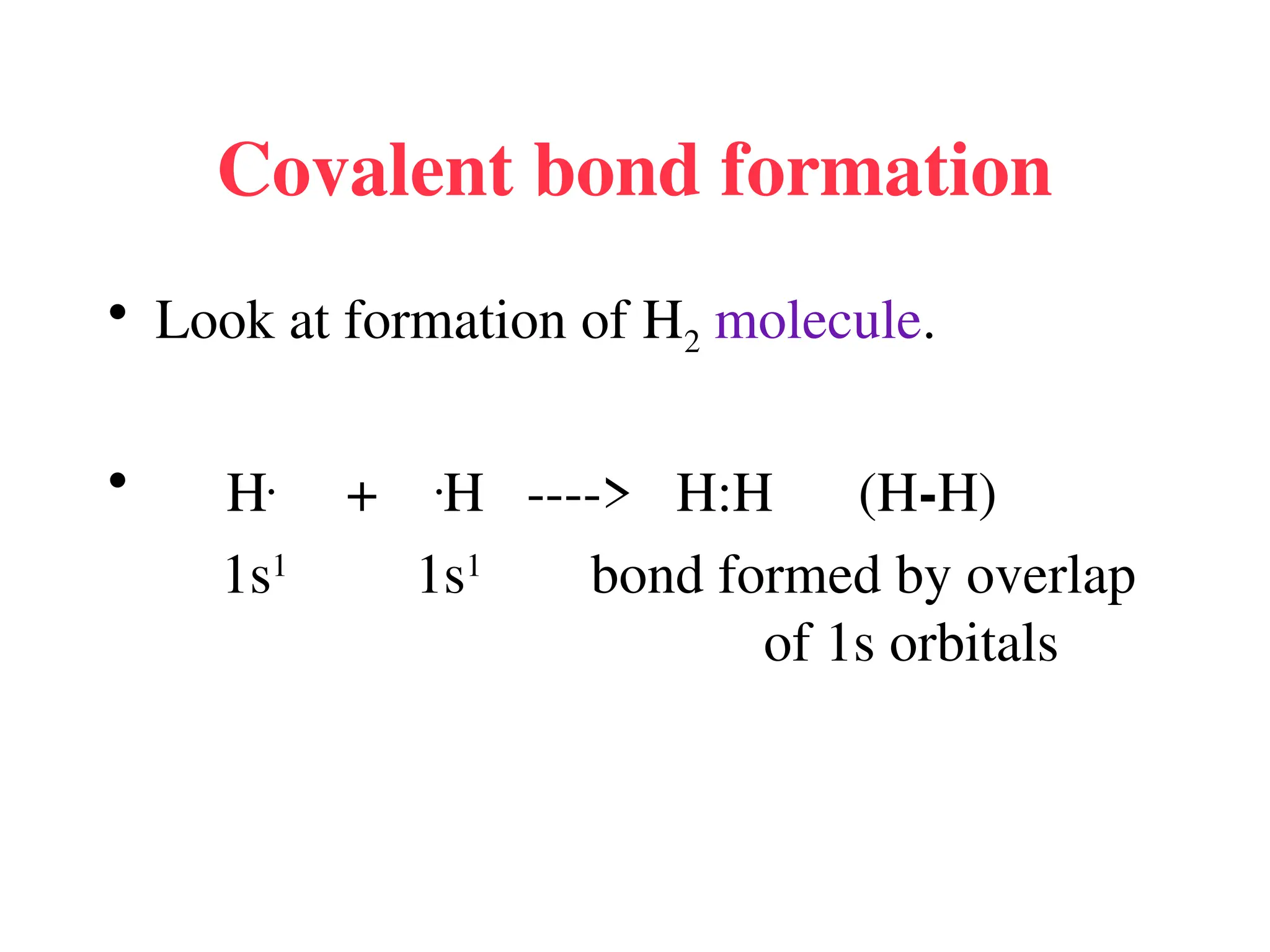
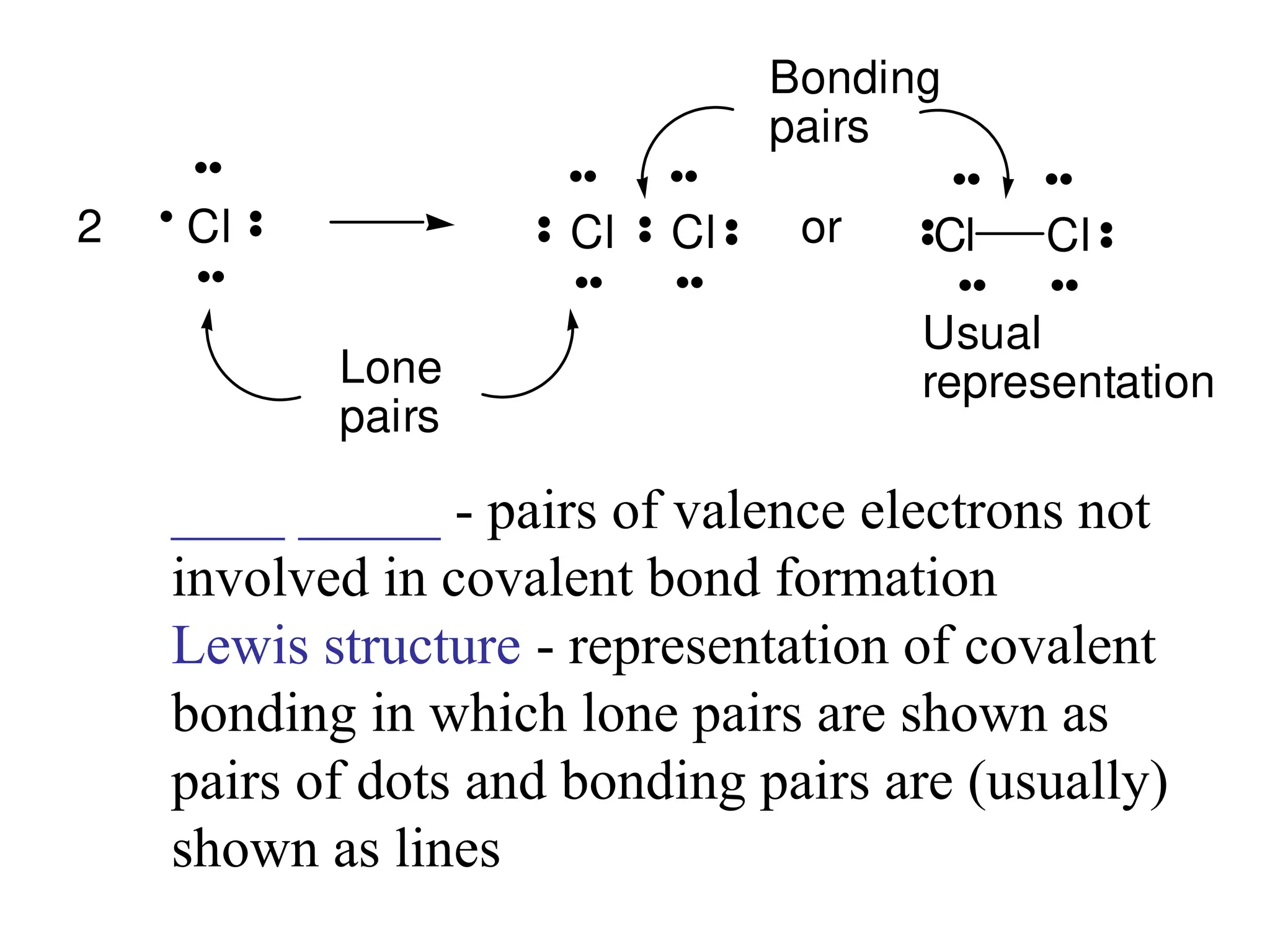
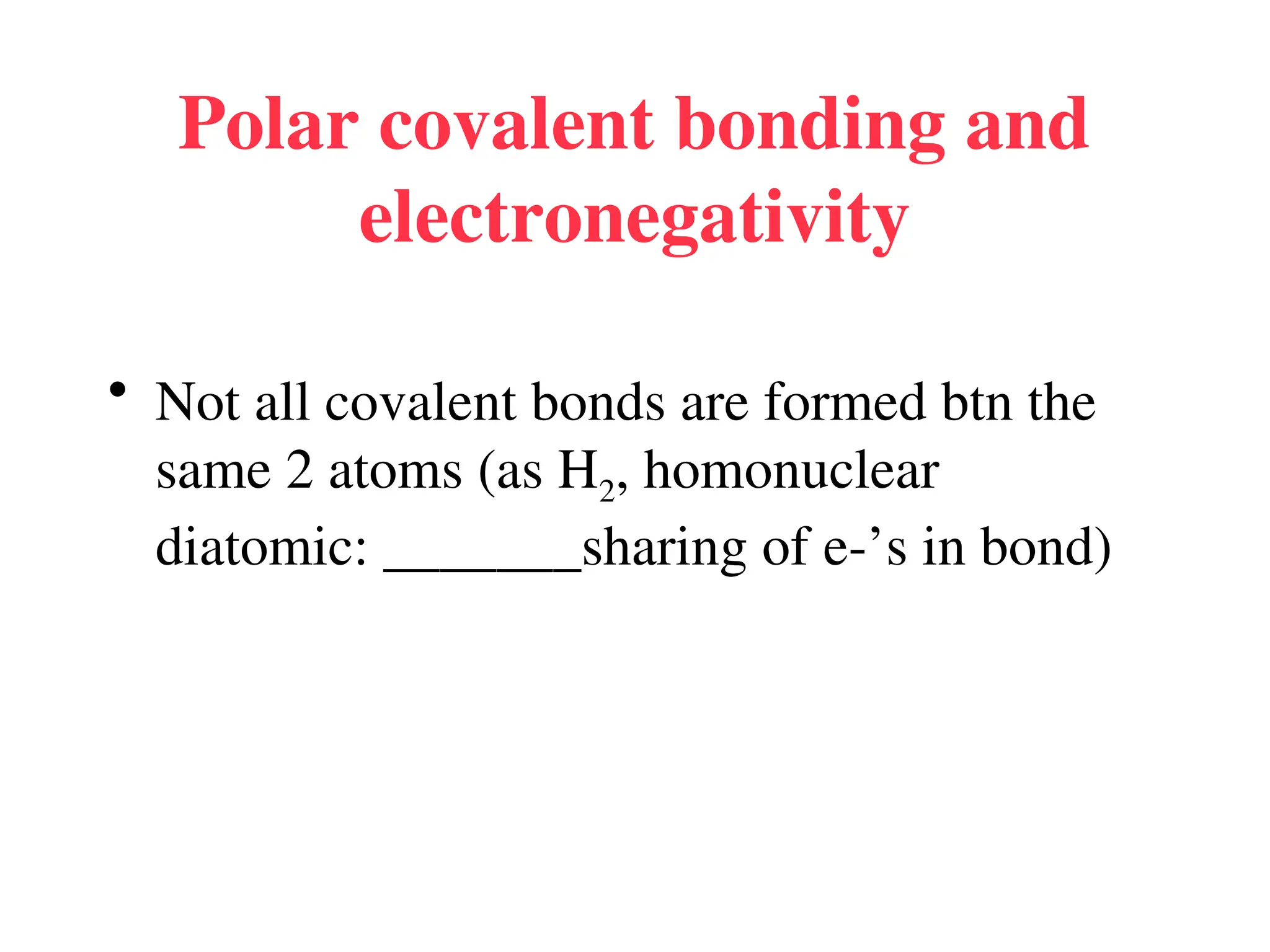
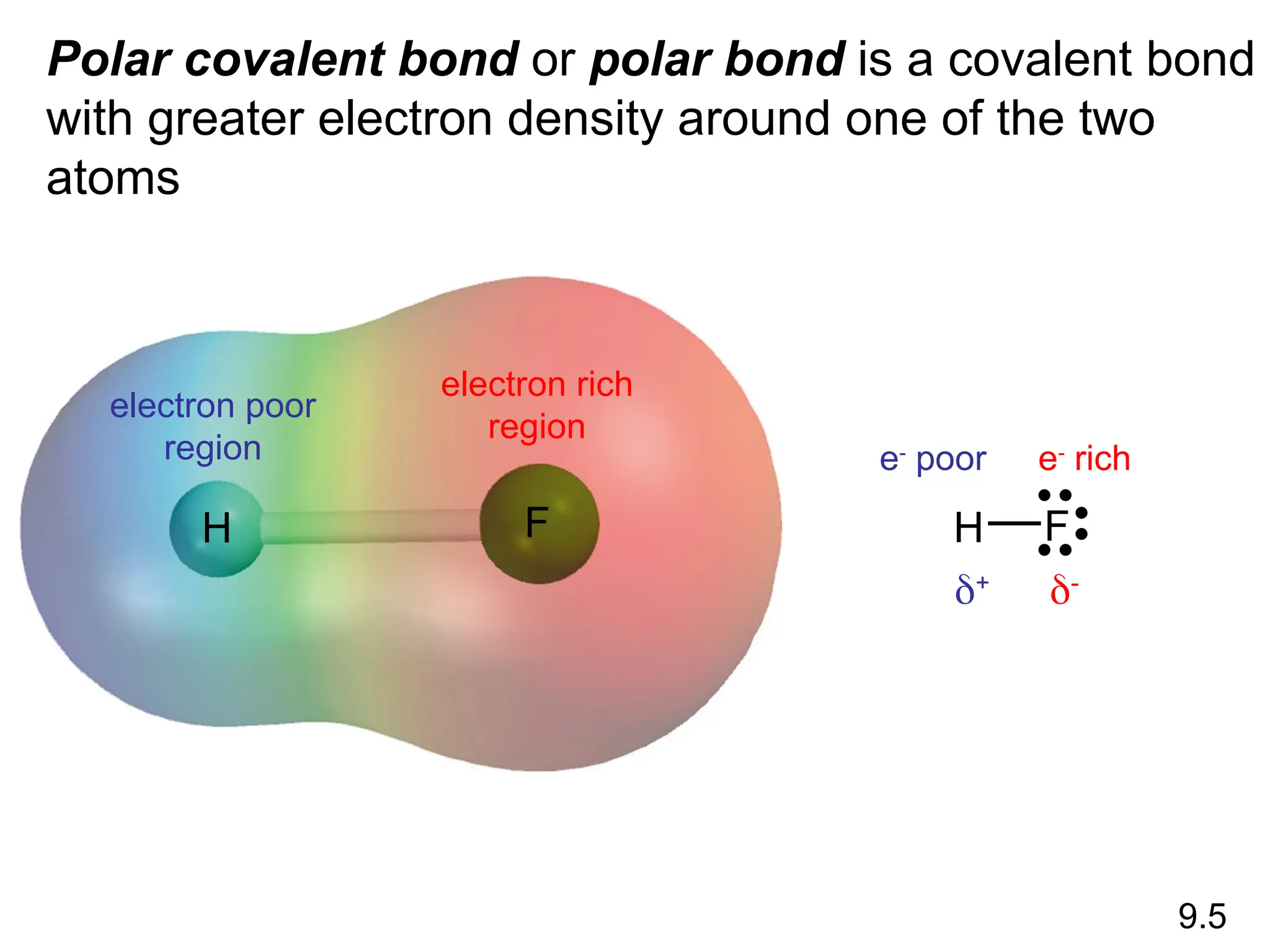
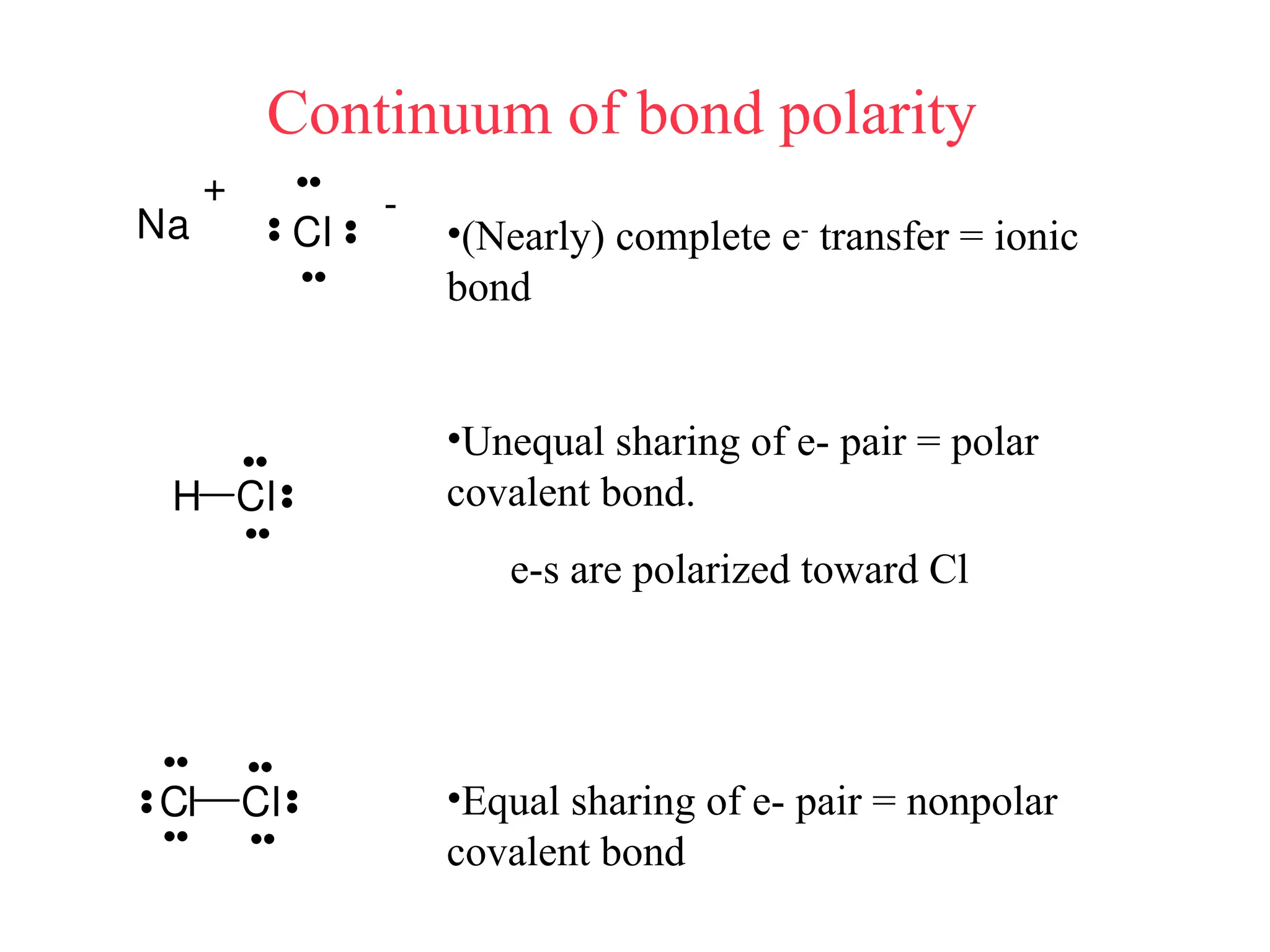
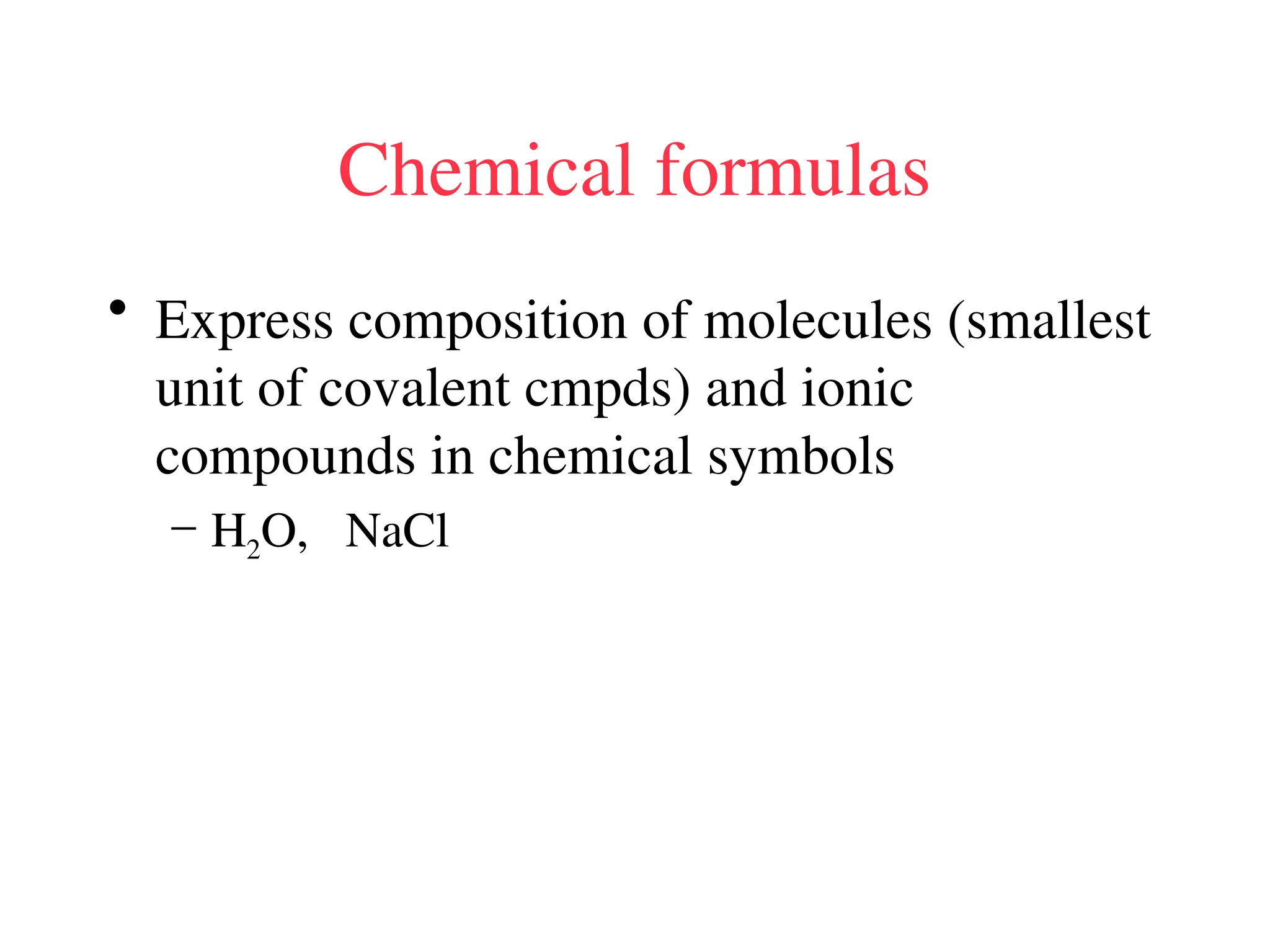
• Remember covalent cmpds--
• A _________ is the smallest unit of a covalent
cmpd that retains the characteristics of the cmpd.
Molecule - two or more atoms in a definite
arrangement held together by chemical bonds.
(H2O, Cl2) [Cl2 is considered a molecule but not a
cmpd]
• Molecular cmpds exist as](https://image.slidesharecdn.com/120ch04-240901093637-26e76639/75/Structure-and-properties-of-ionic-and-covalent-compounds-ppt-59-2048.jpg)