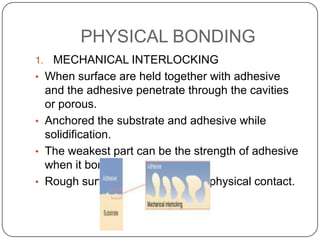
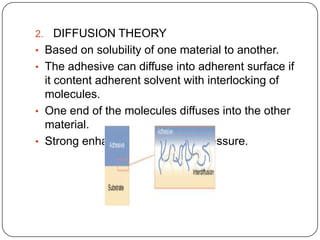
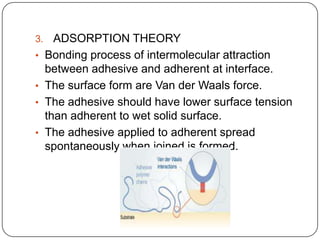
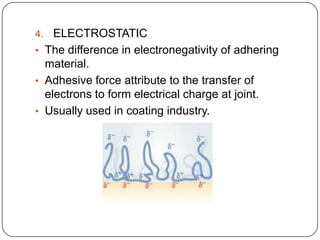
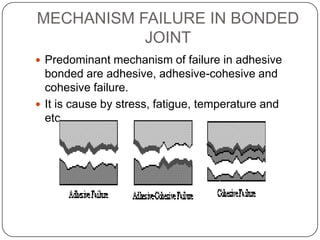
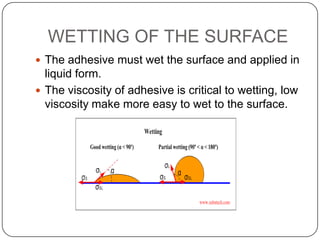
This document discusses polymerization and adhesion mechanisms. It defines polymers, monomers, homopolymers and copolymers. It then describes the main mechanisms of adhesion - chemical bonding through primary and secondary bonds, and physical bonding through mechanical interlocking, diffusion, adsorption, and electrostatic attraction. It also briefly discusses mechanisms of bonded joint failure and criteria for good adhesive bonds such as wetting of the surface.