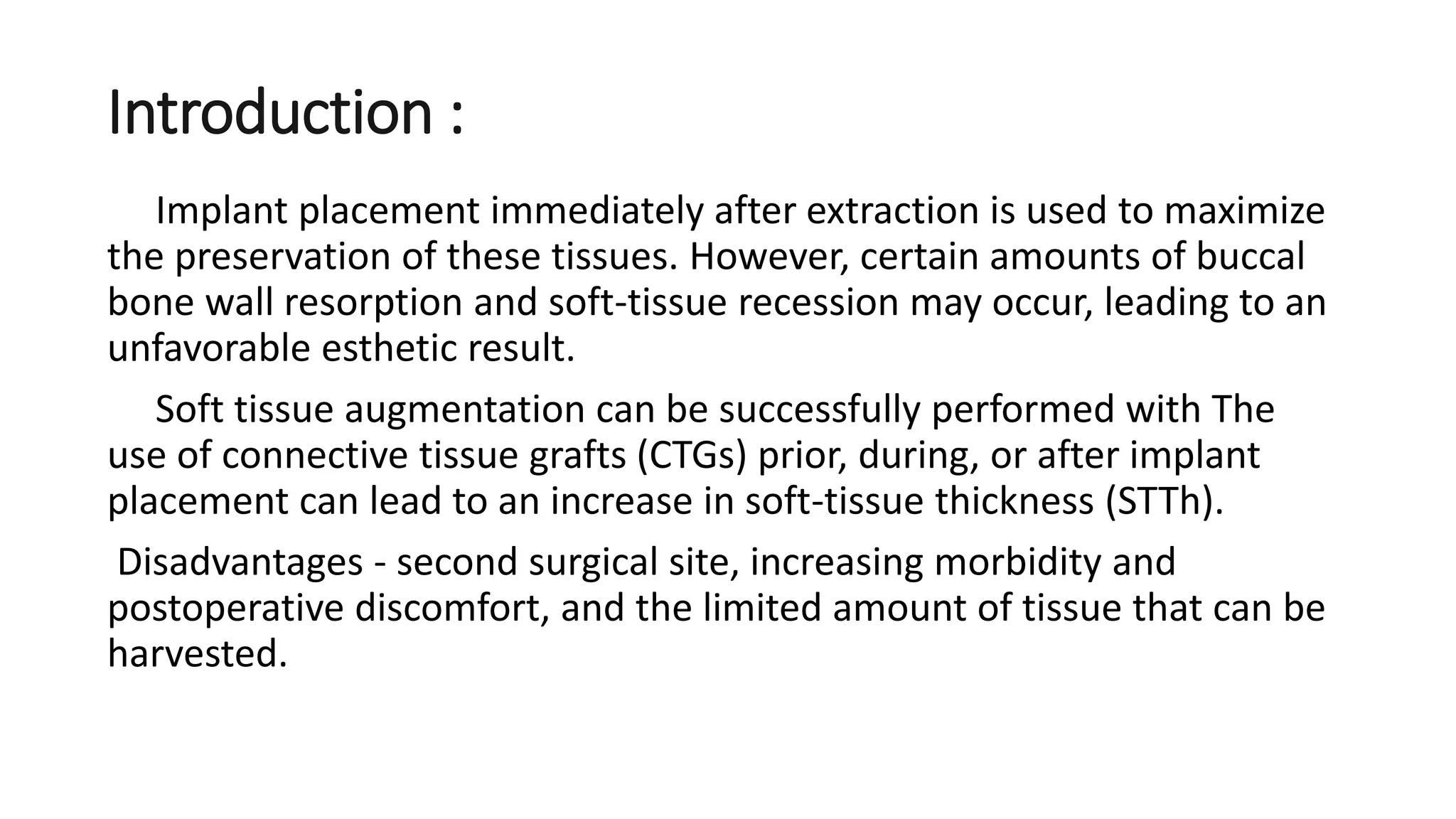
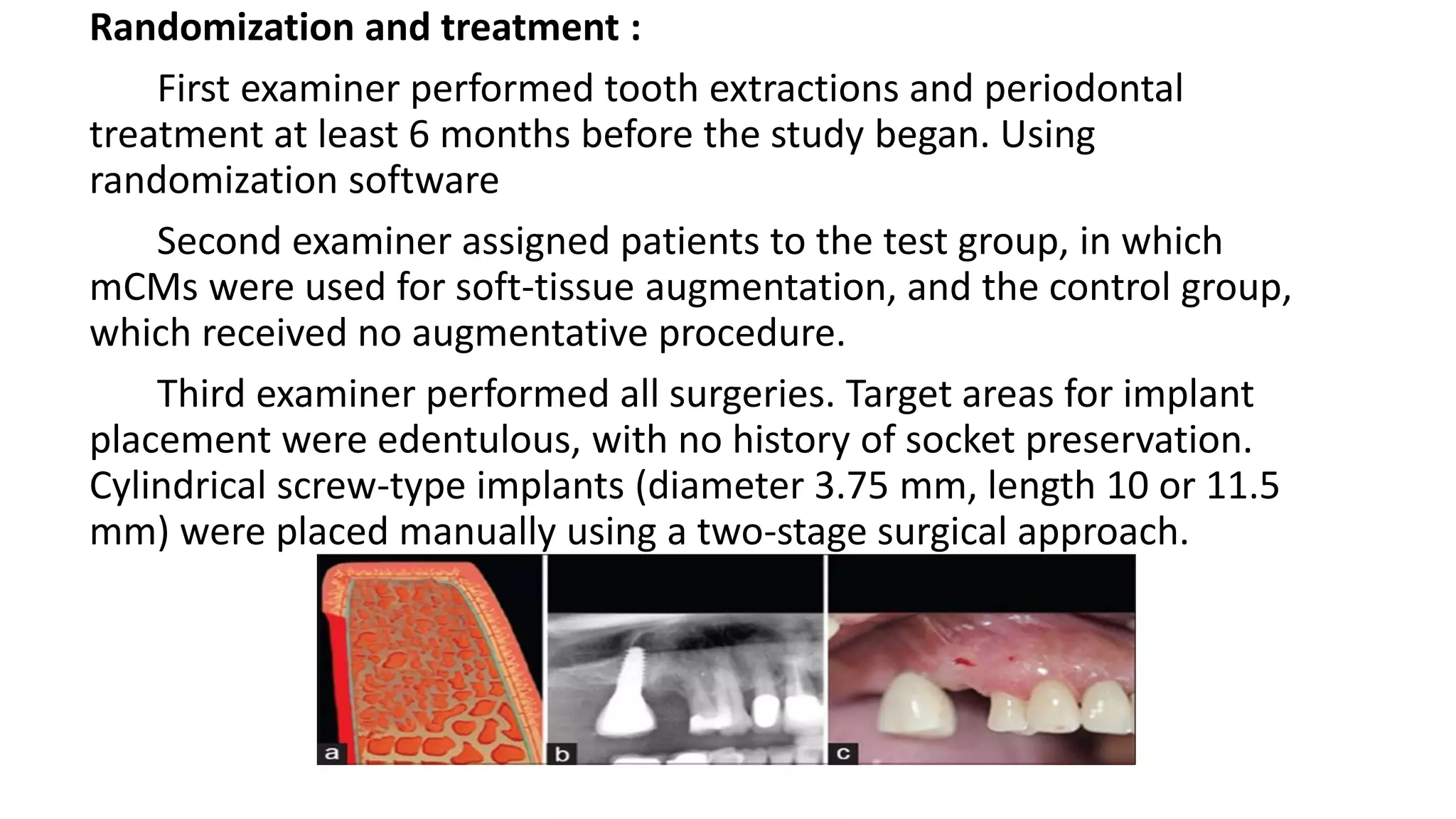
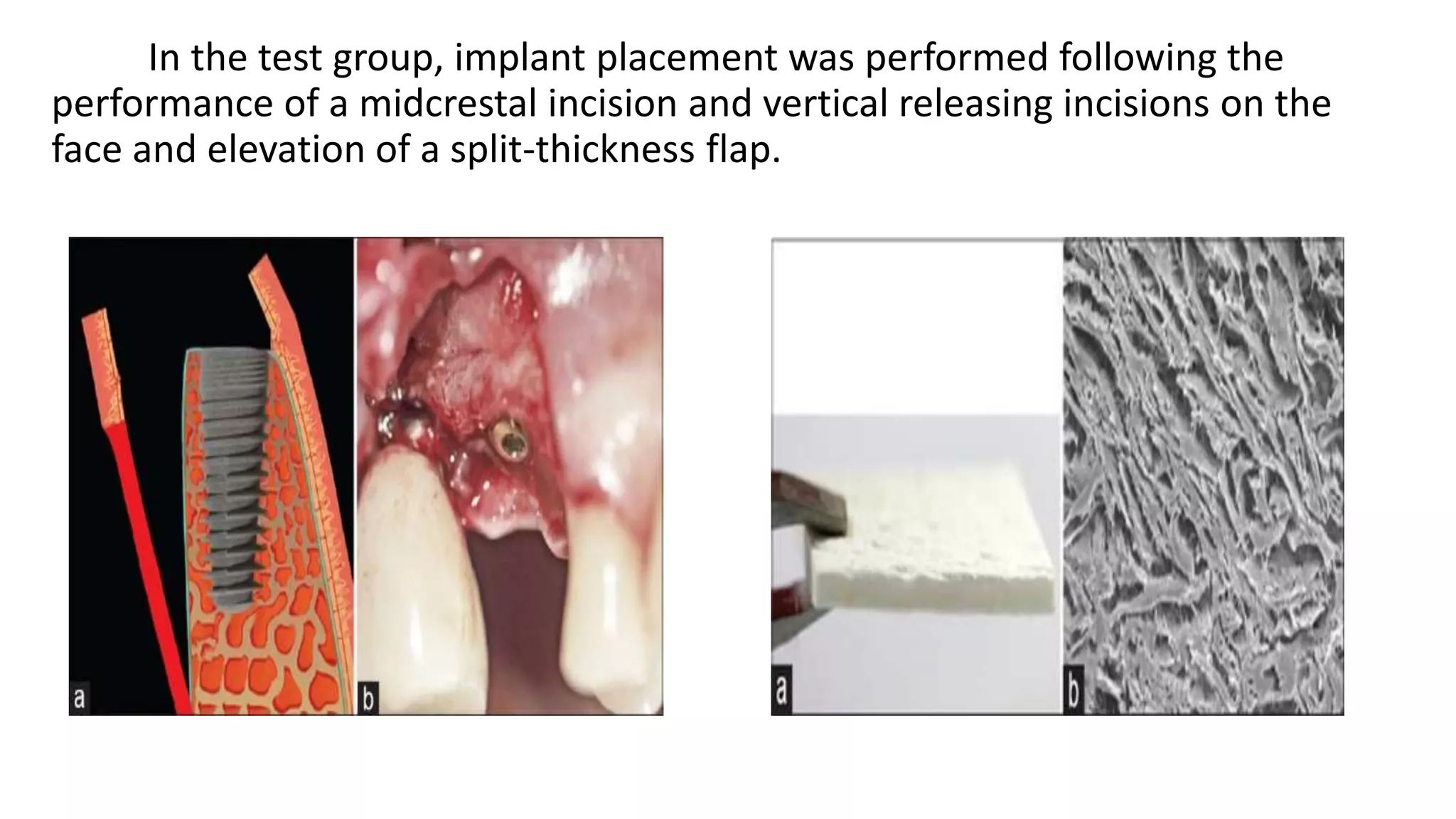
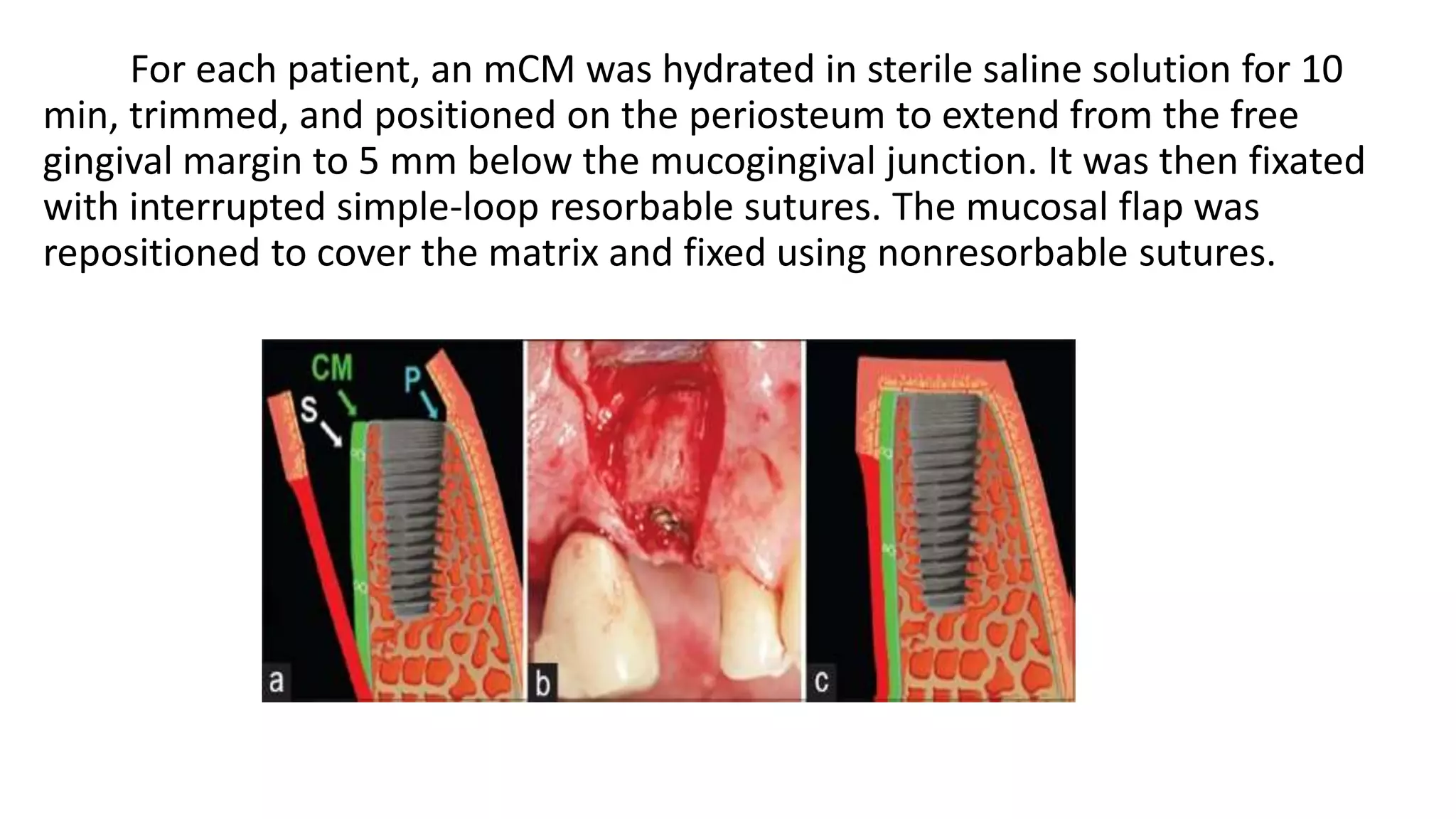
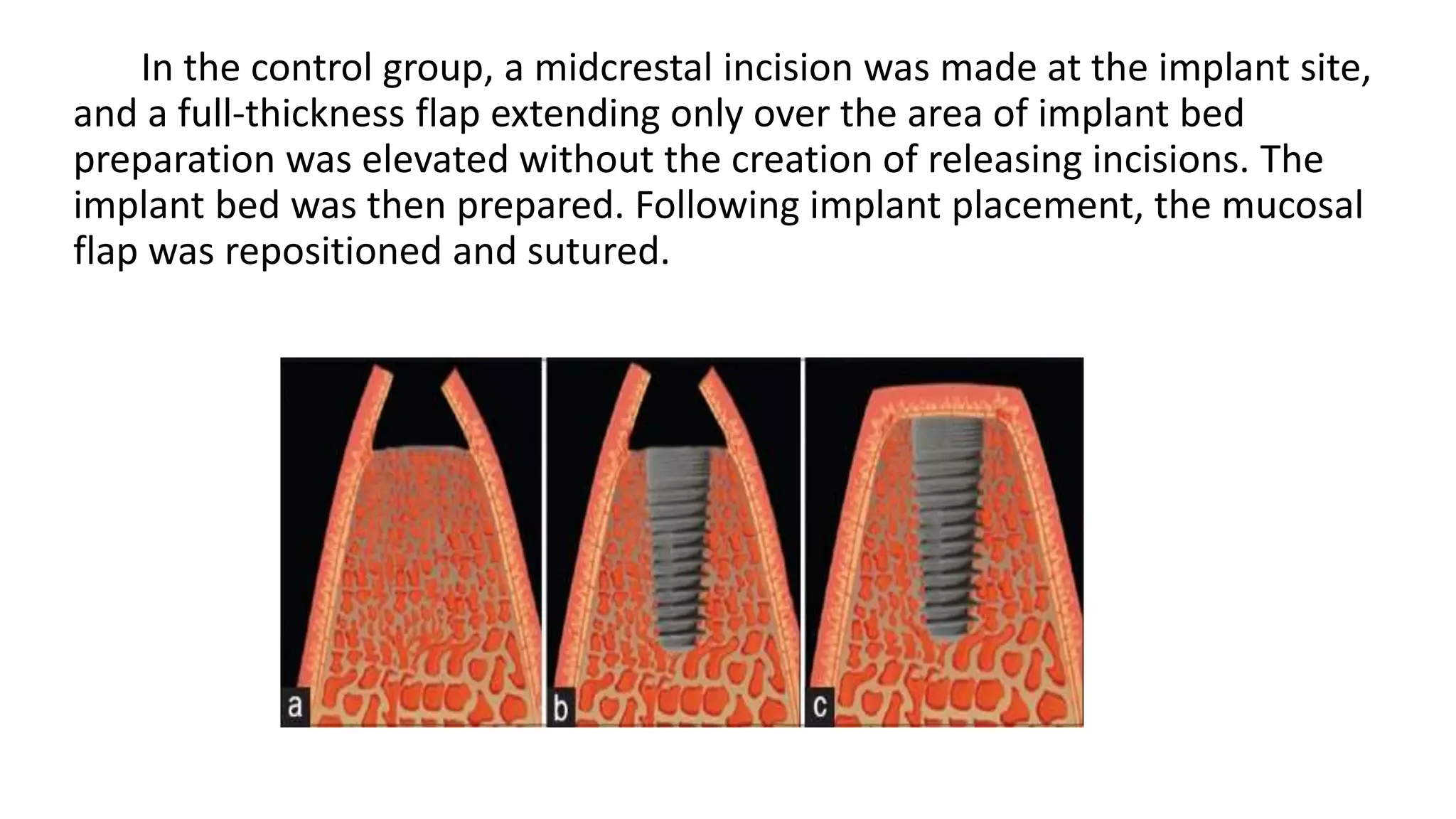
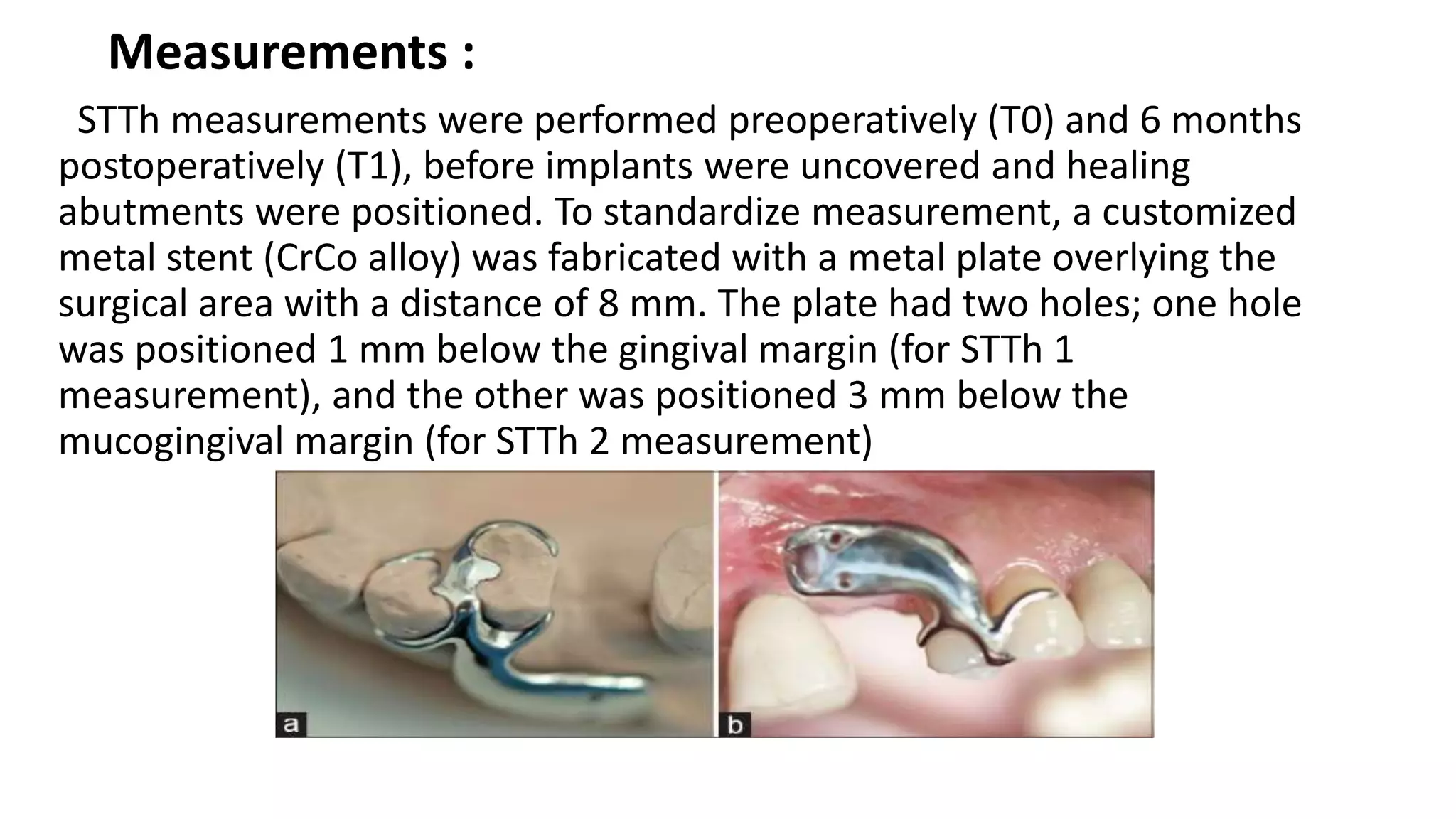
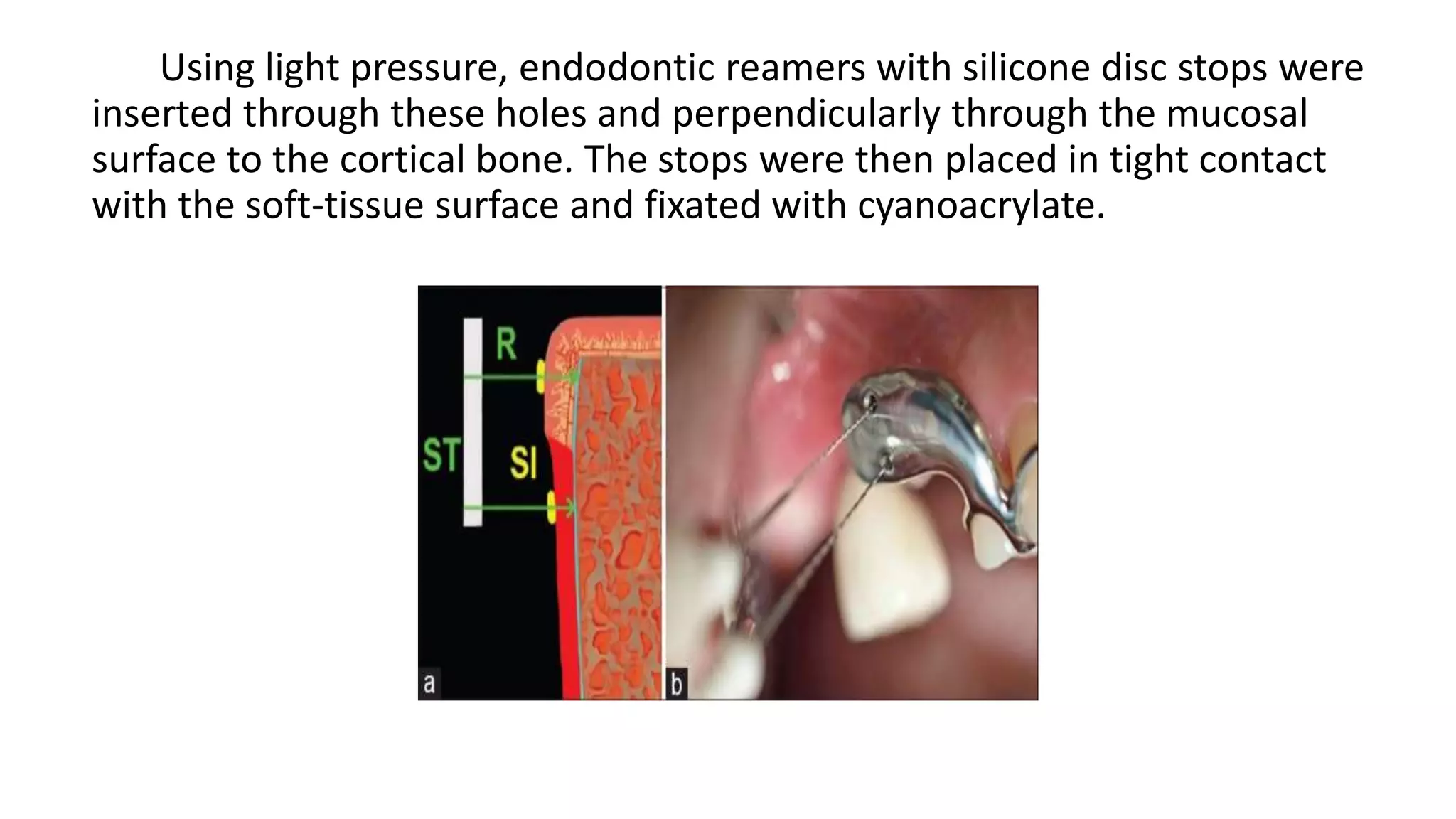
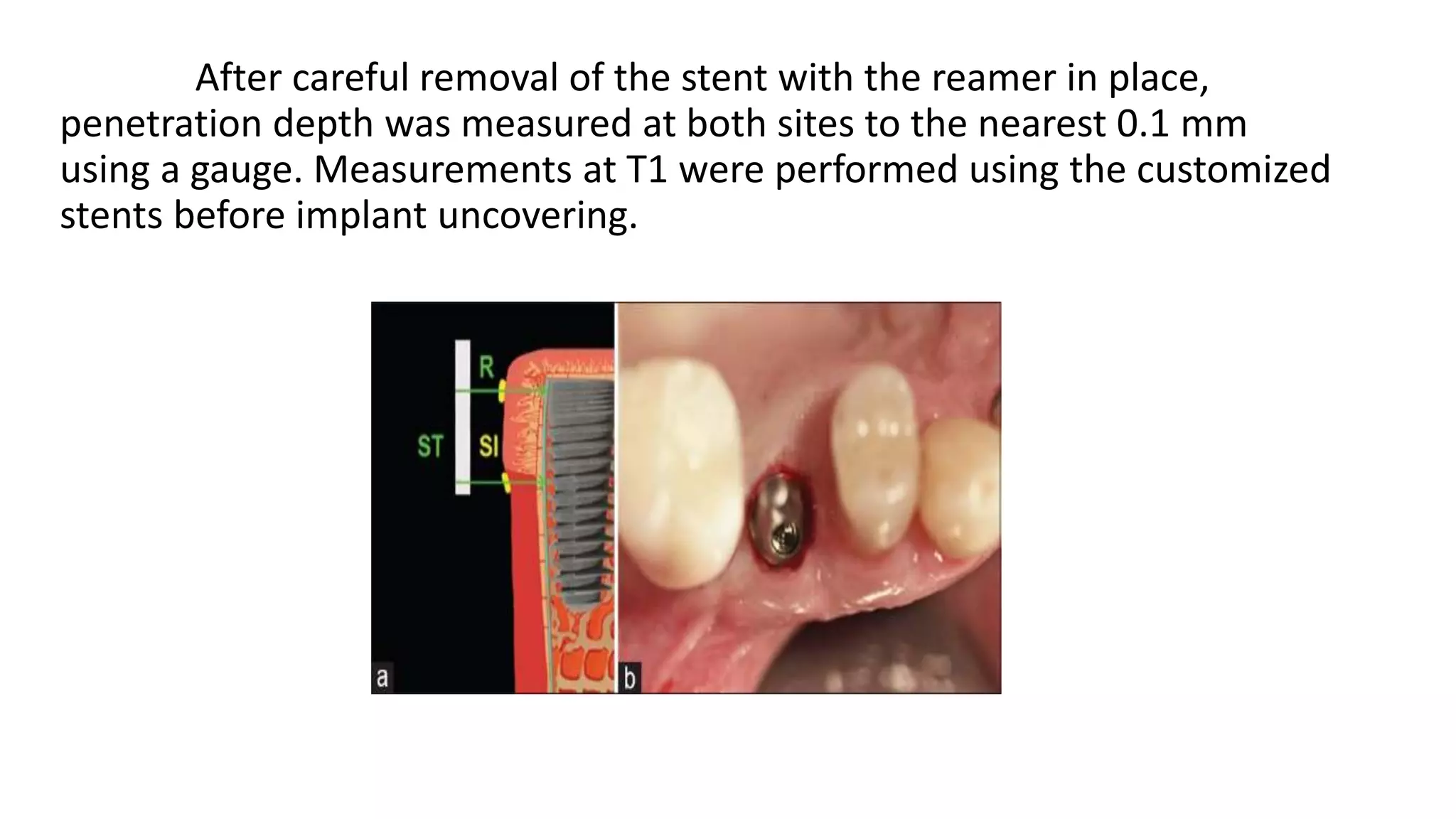
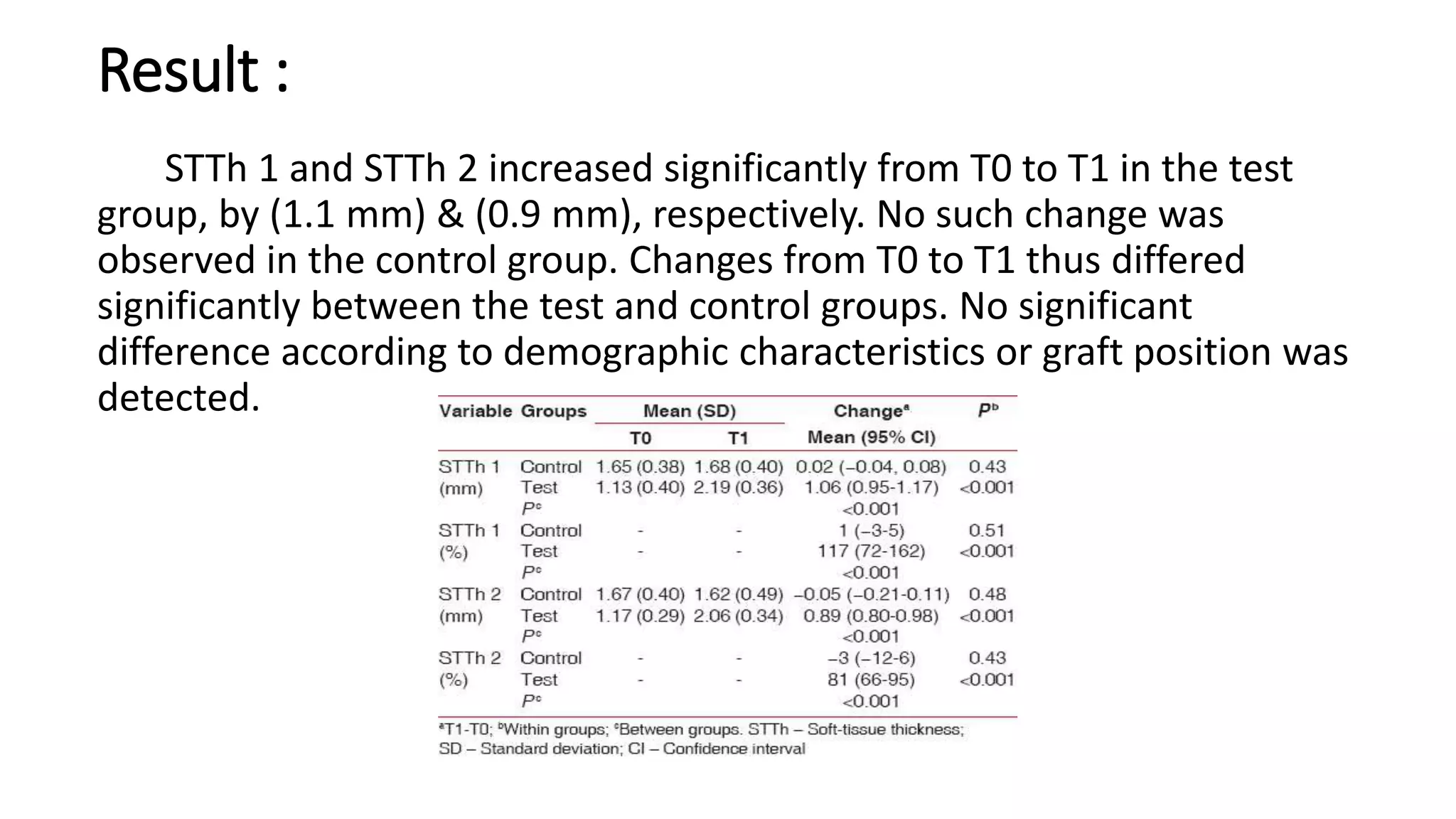
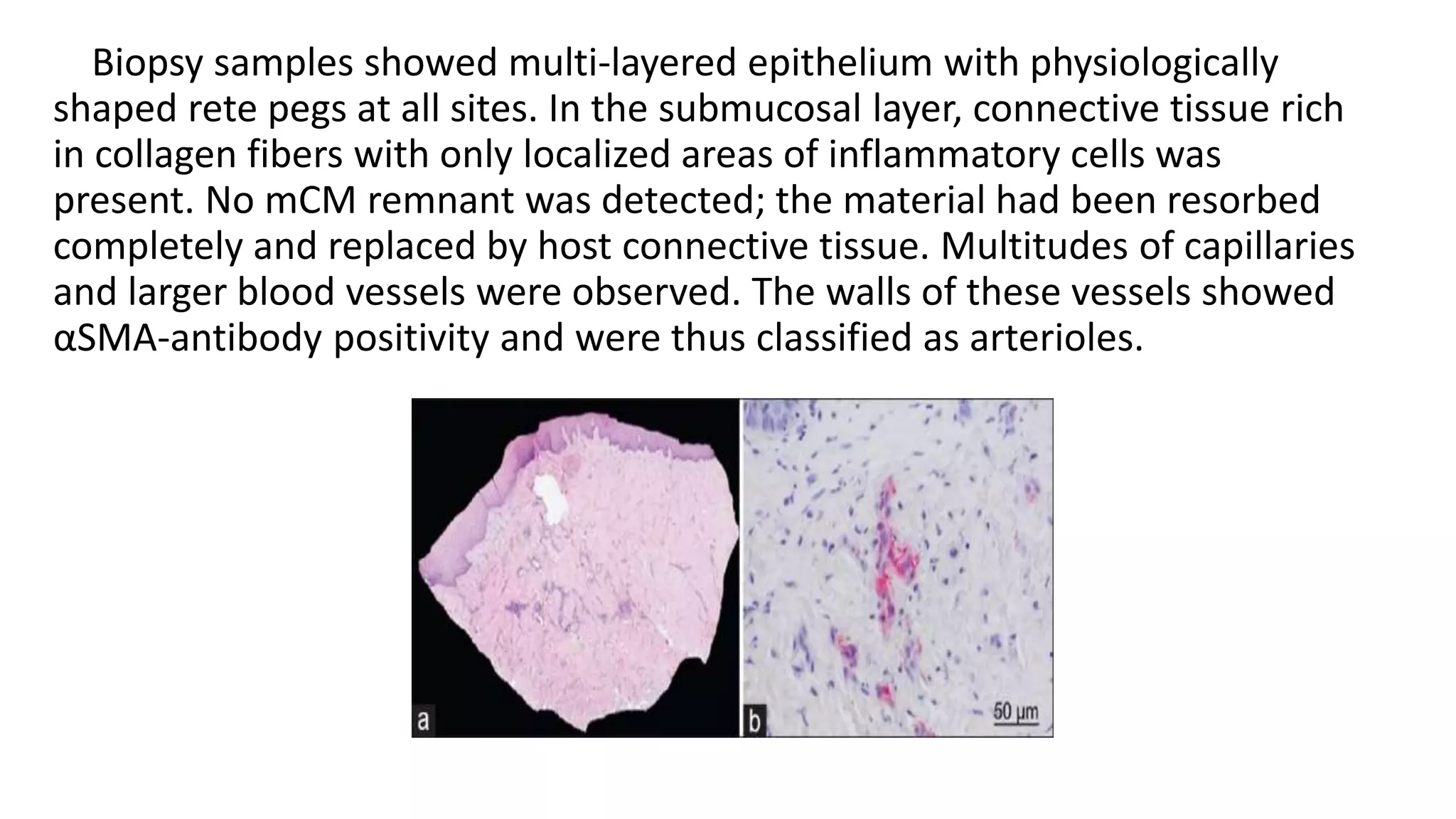
The document summarizes a clinical study that evaluated the use of a collagen matrix (CM) to increase soft tissue thickness around dental implants. In the study, 27 patients received either a CM graft (test group) or no augmentation (control group) during implant placement. Measurements before and 6 months after found that the soft tissue thickness increased significantly in the test group by 1.1 mm and 0.9 mm, but did not change in the control group. Histological analysis showed the CM had degraded and been replaced by new host soft tissue rich in collagen and blood vessels. The study concluded that CM is an effective alternative to connective tissue grafts for augmenting peri-implant soft tissue thickness.