This document provides information about colorectal cancer, including:
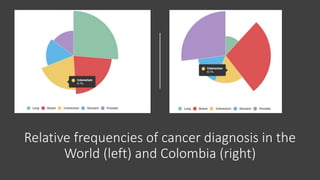
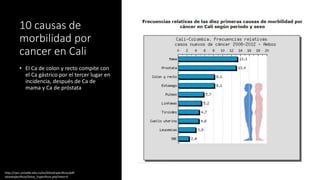
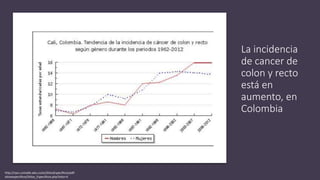
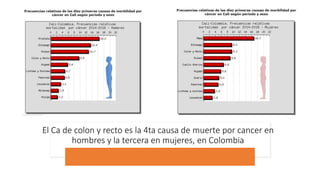
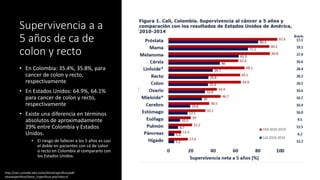
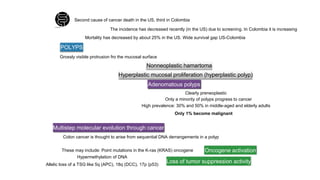
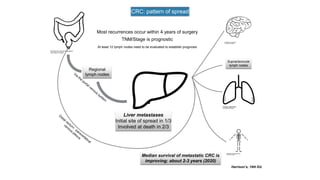
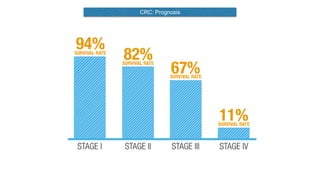
- Colorectal cancer is the third leading cause of cancer death in Colombia. Survival rates are much lower in Colombia than the US.
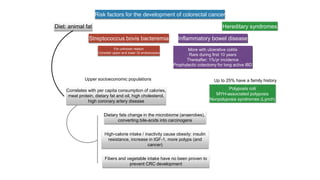
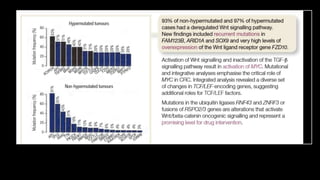
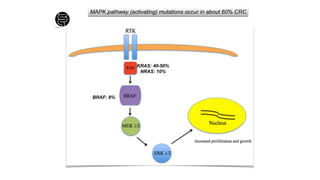
- Risk factors include diet high in fat and low in fiber, obesity, inflammatory bowel disease, family history, and hereditary syndromes.
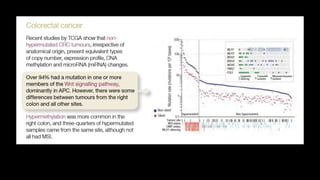
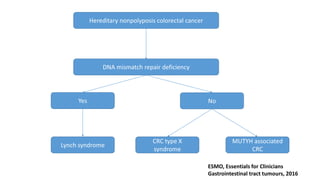
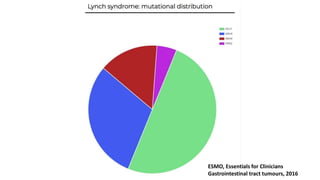
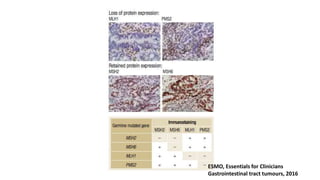
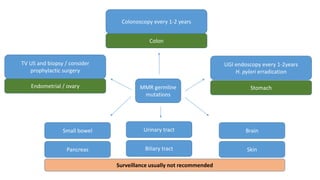
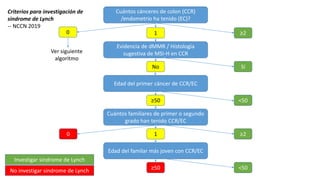
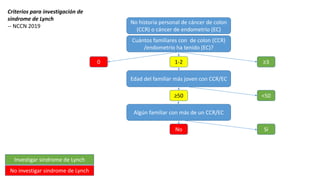
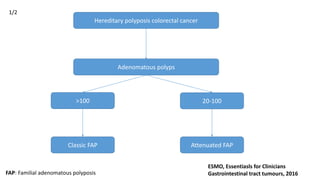
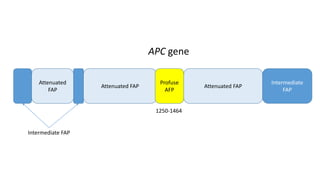
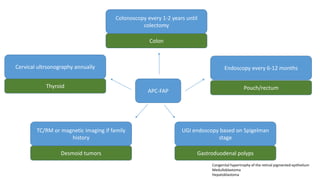
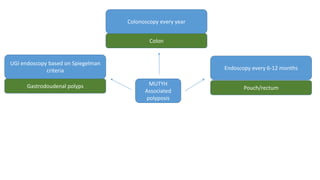
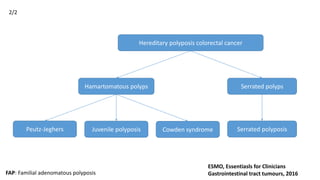
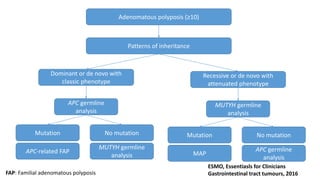
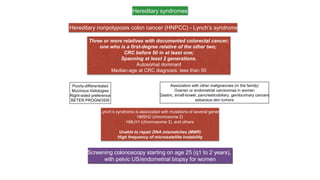
- Hereditary syndromes associated with colorectal cancer include Lynch syndrome, familial adenomatous polyposis (FAP), and MUTYH-associated polyposis (MAP).
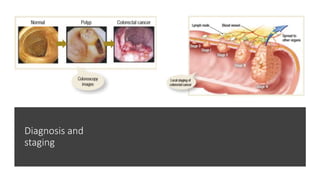
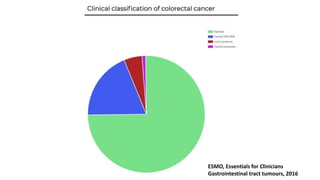
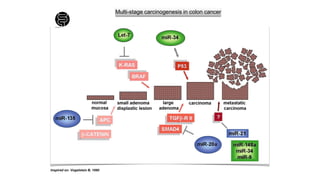
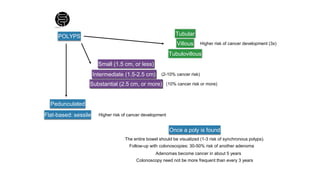
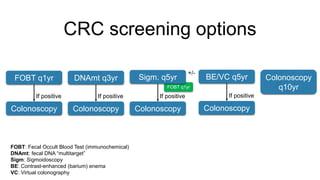
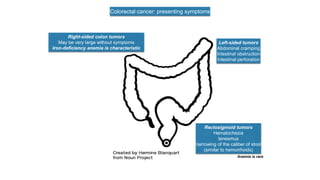
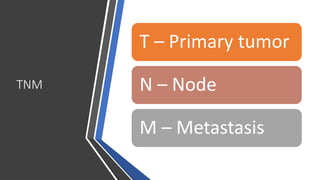
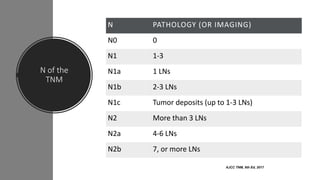
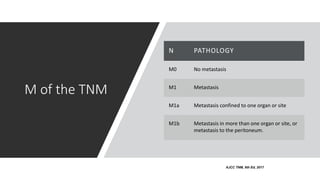
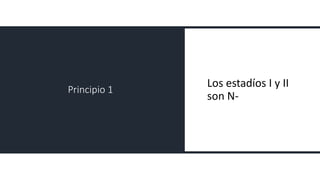
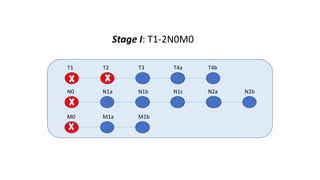
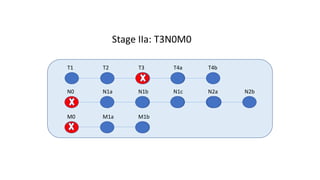
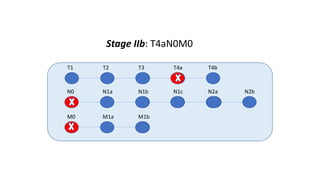
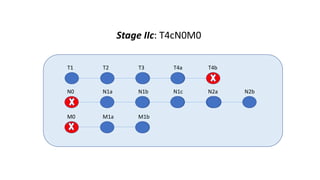
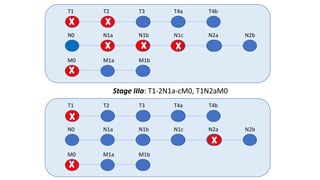
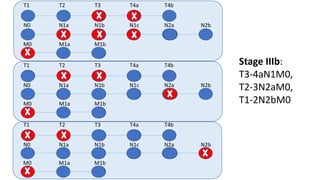
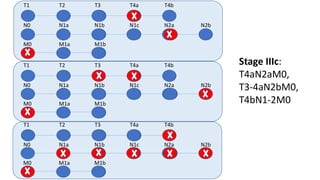
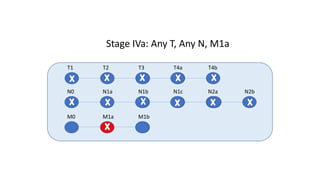
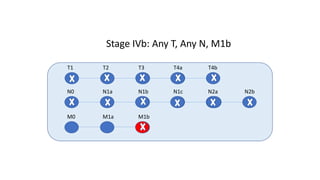
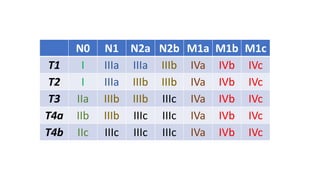
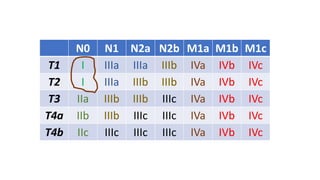
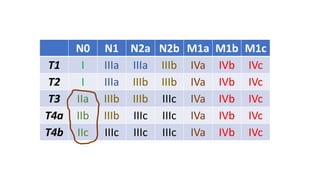
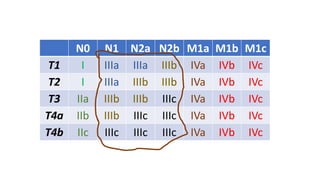
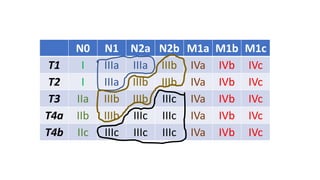
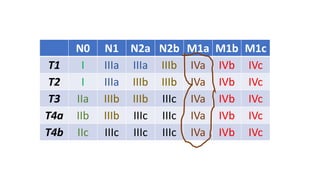
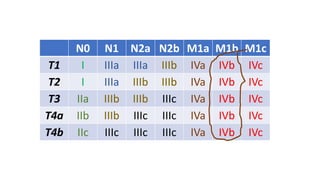
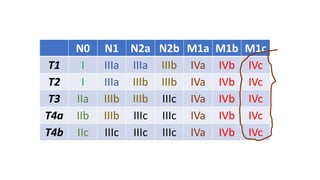
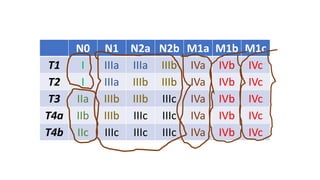
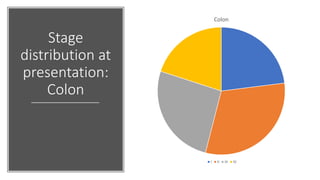
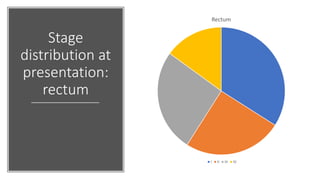
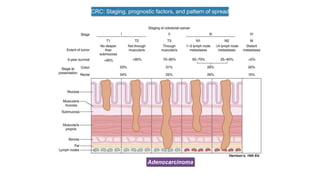
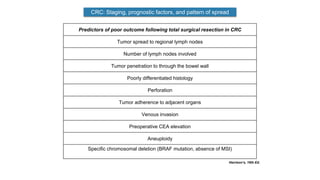
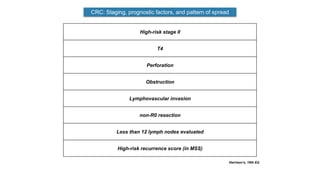
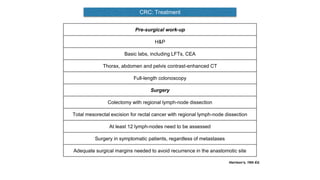
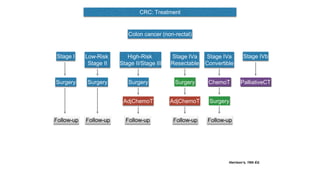
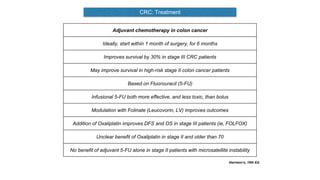
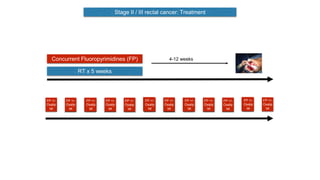
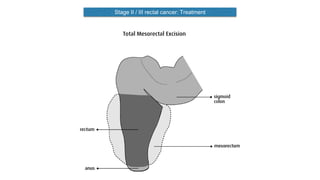
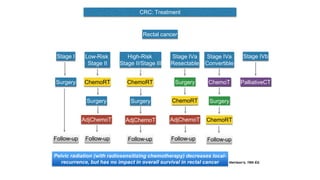
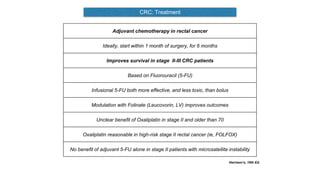
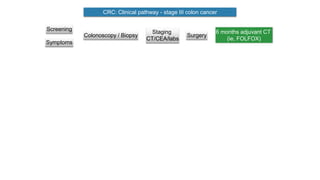
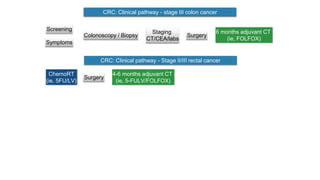
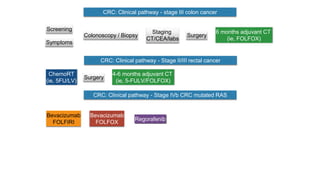
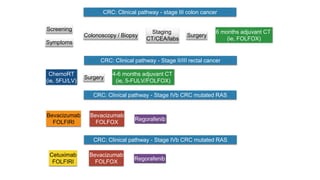
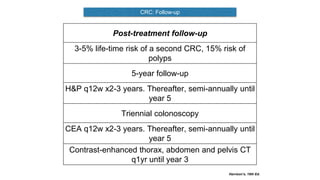
- Diagnosis involves screening via colonoscopy to detect polyps which can be removed to prevent cancer development. Staging determines cancer extent and guides treatment.