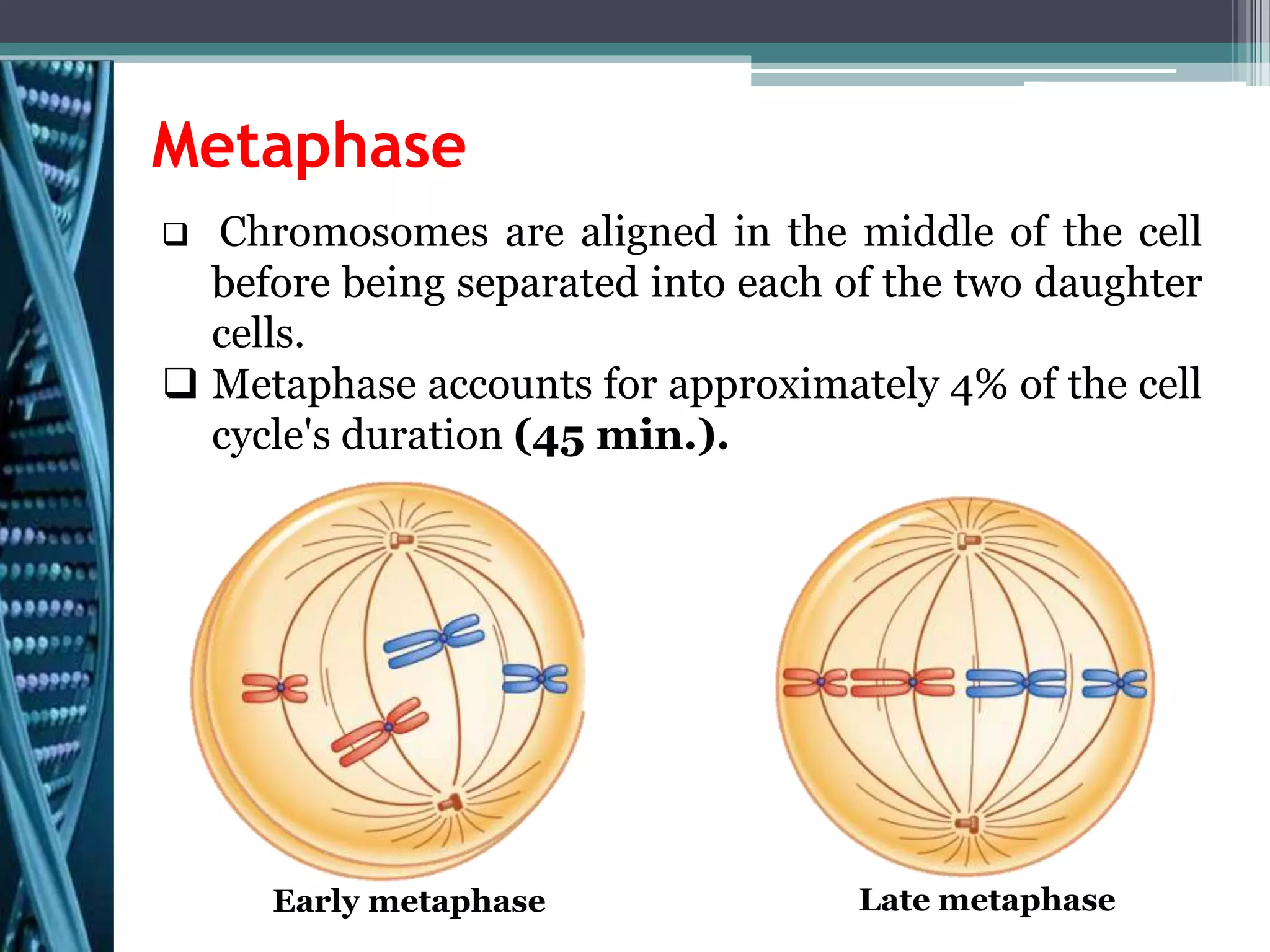
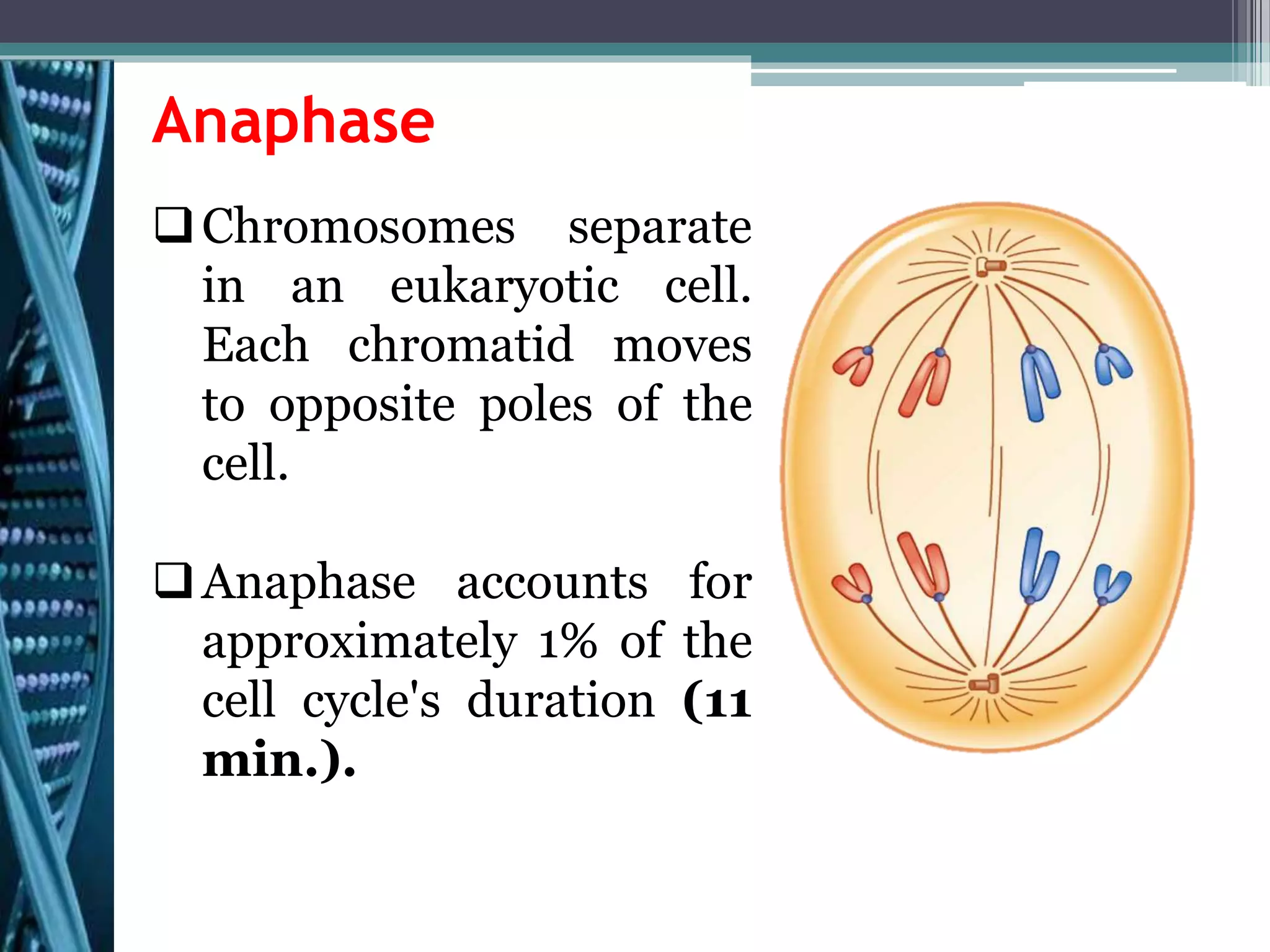
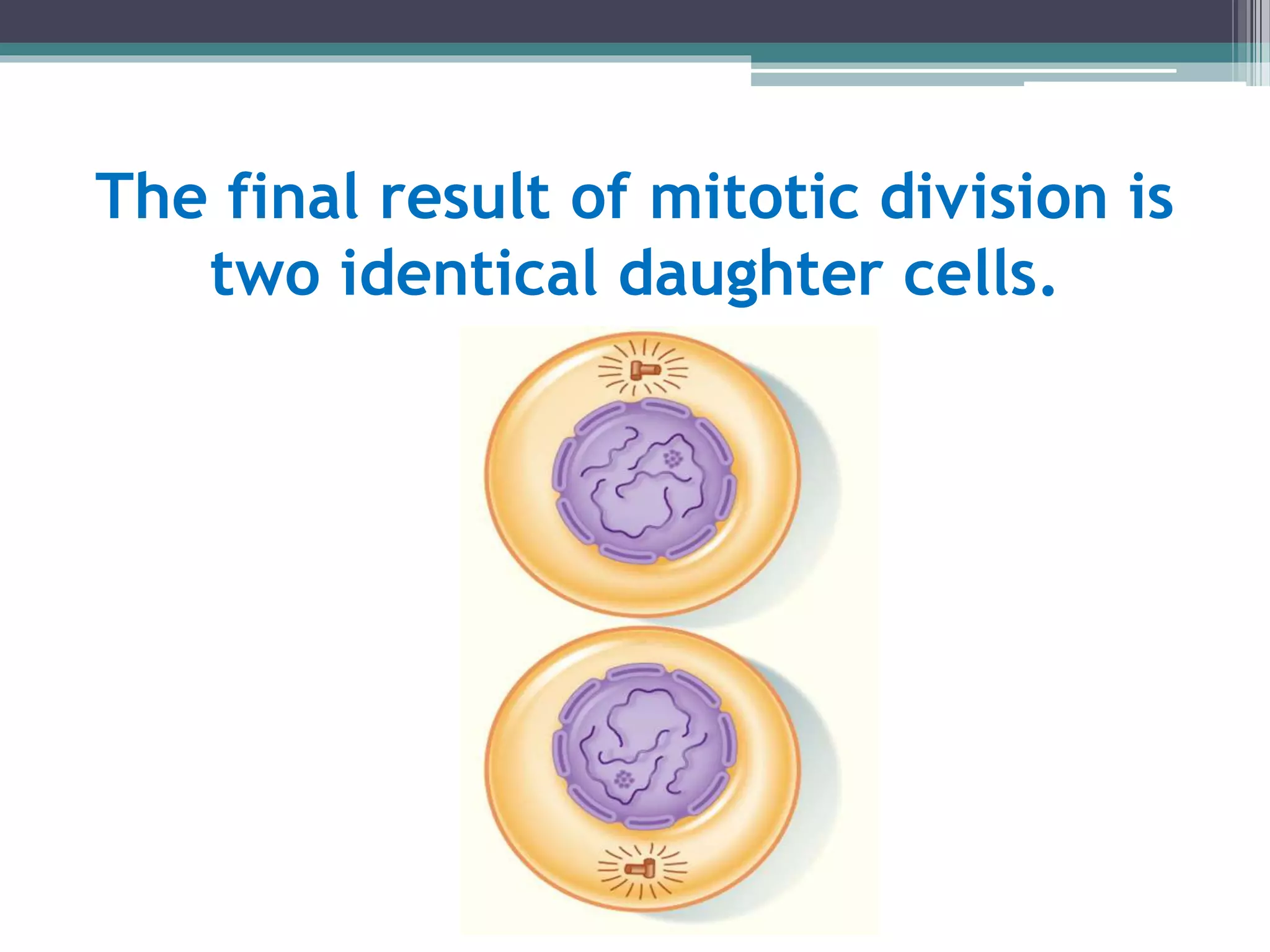
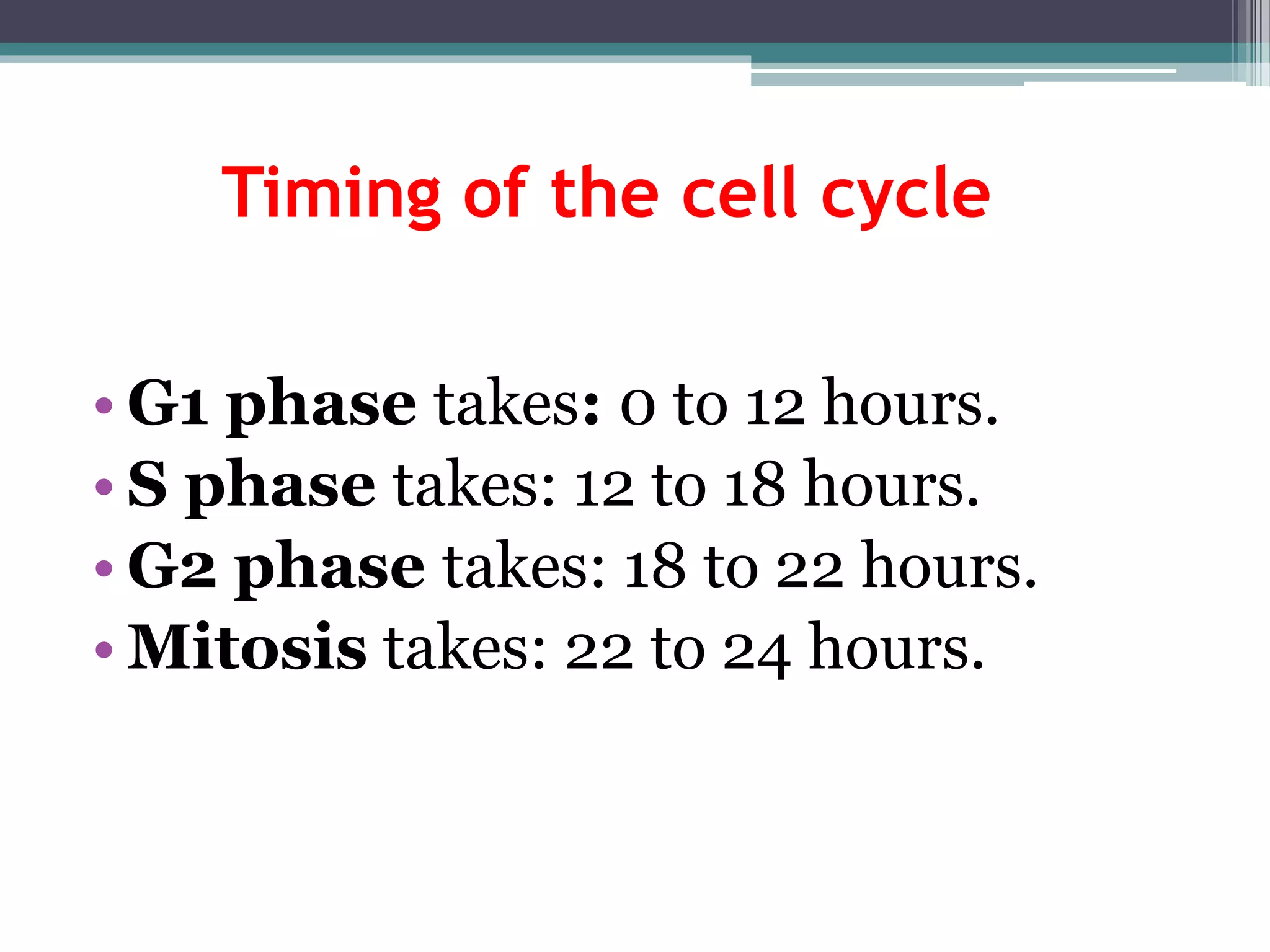
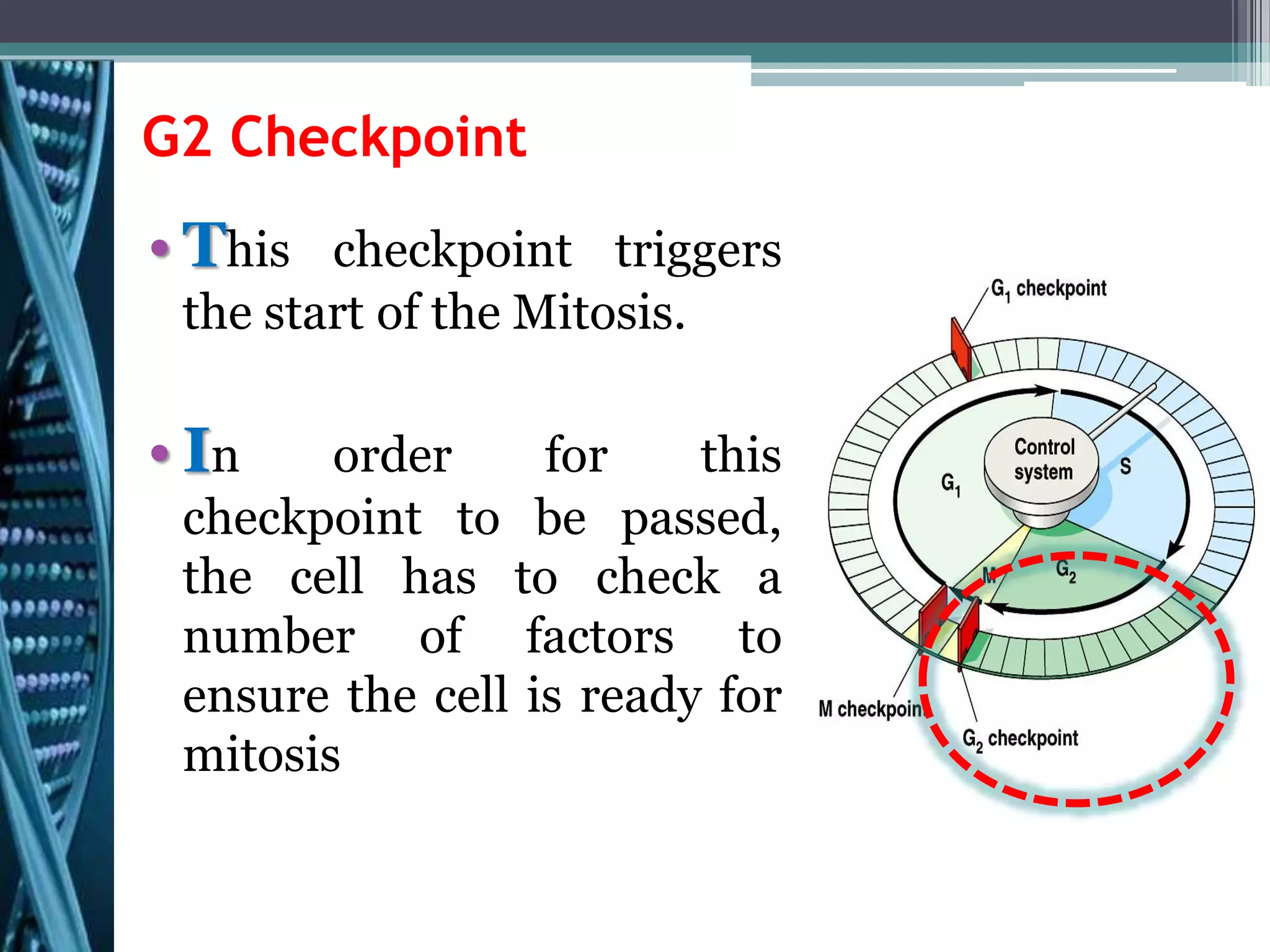
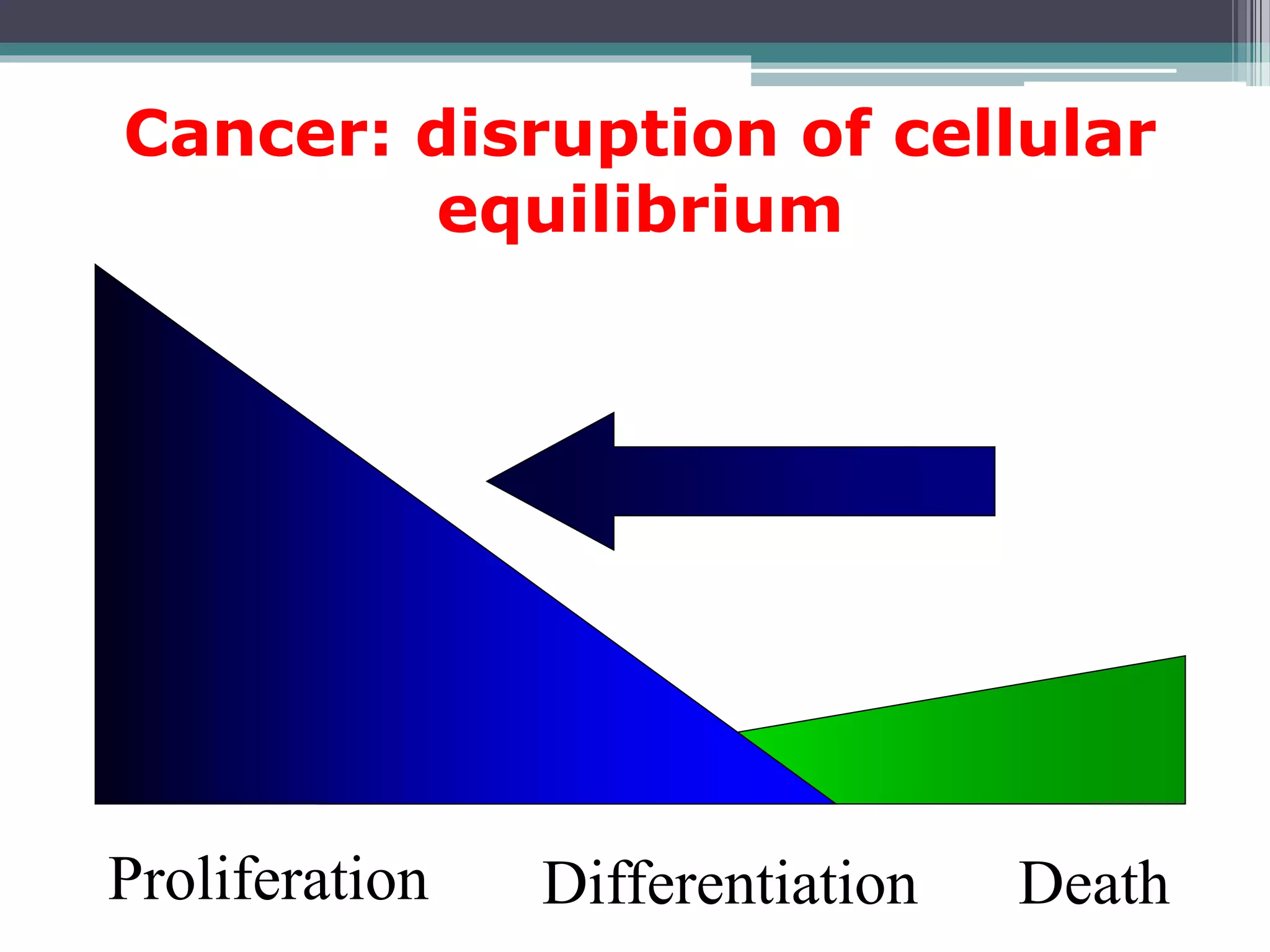
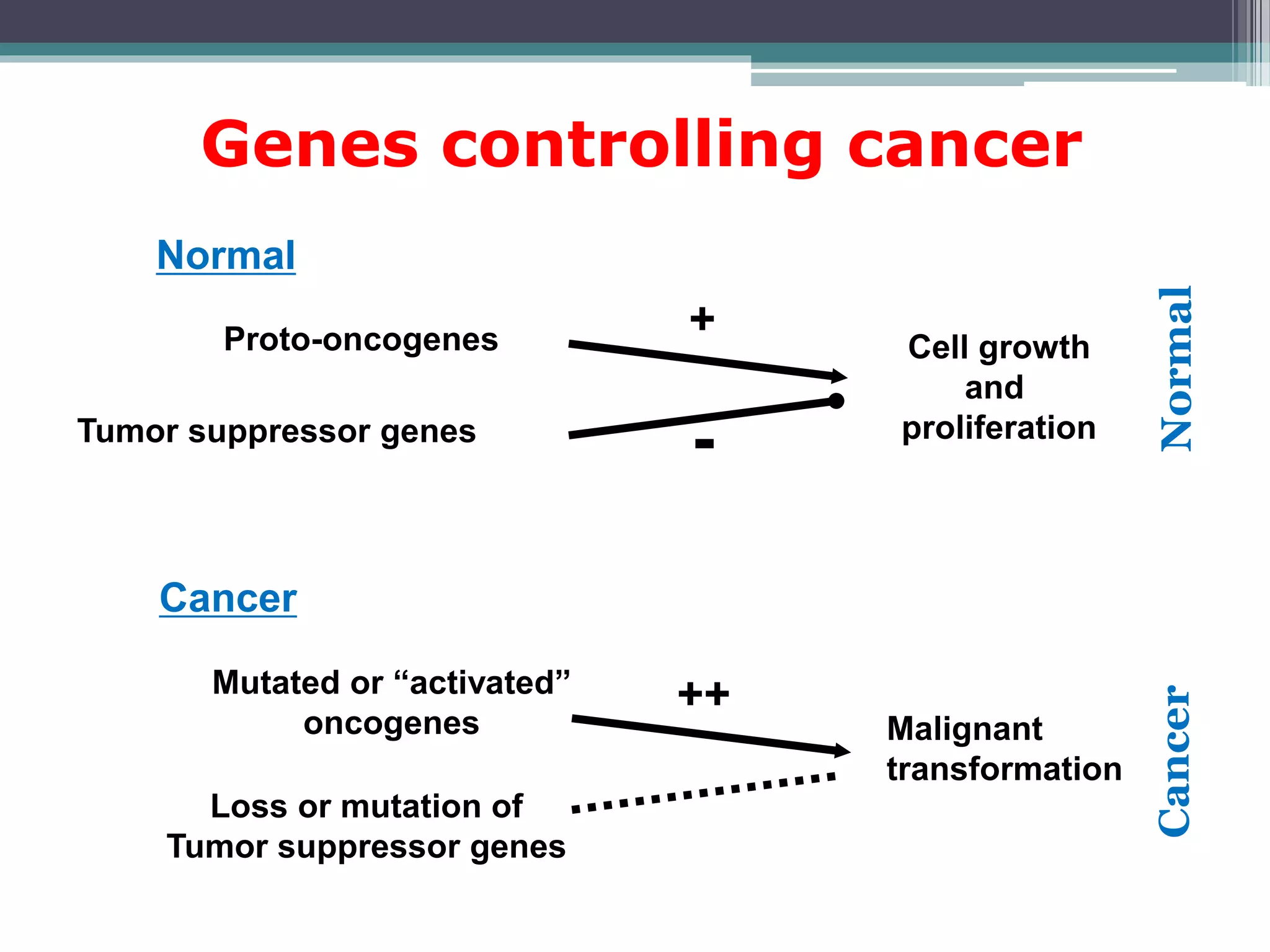
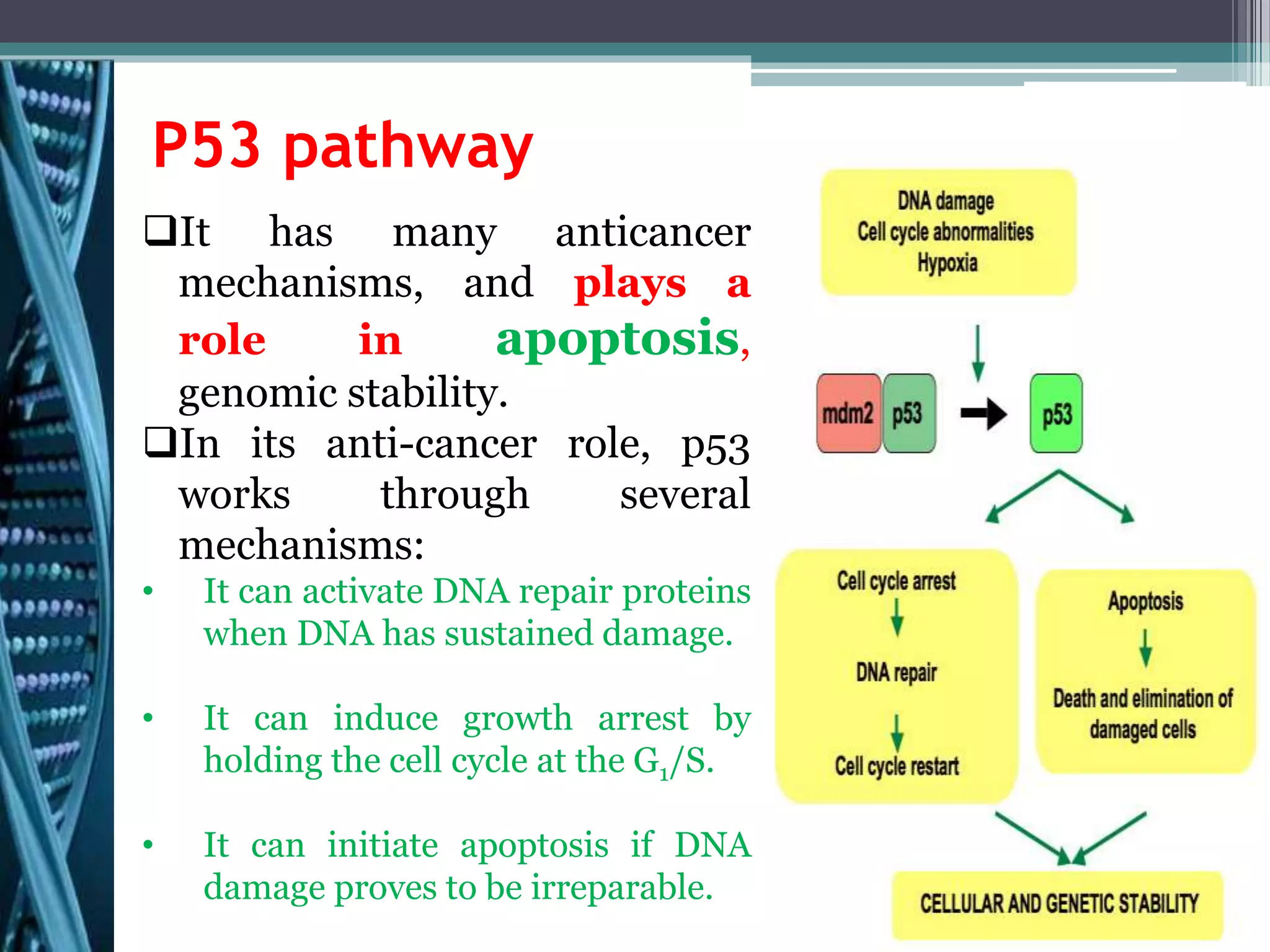
Cell cycle checkpoints play an important role in ensuring the proper progression of the cell cycle and preventing unregulated cell growth. Checkpoints at the G1/S and G2/M transitions verify that the cell is ready to progress prior to DNA replication or mitosis. Loss of checkpoint function can allow mutations to accumulate, potentially leading to carcinogenesis. Cancer arises through genetic changes that disrupt the normal balance between cell proliferation, differentiation, and apoptosis. This includes mutations in oncogenes and tumor suppressor genes such as p53, which normally function to regulate the cell cycle and cell growth.