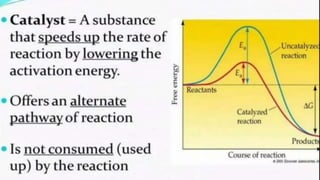
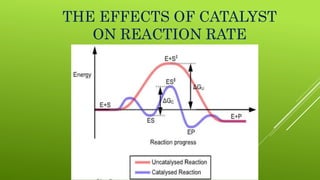
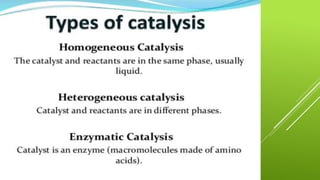
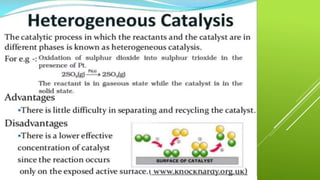
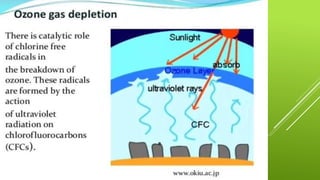
The document discusses how catalysts can increase the rate of chemical reactions. It explains that catalysts provide an alternative reaction pathway that has a lower activation energy, making it easier for reactions to occur. Catalysts work by speeding up reactions without being used up in the process. Common examples of catalysts mentioned are enzymes in biochemical reactions and substances added to chemical processes to lower their temperature or pressure requirements.