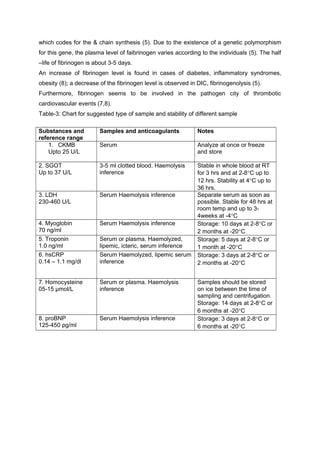
This document discusses various cardiac markers that can be used to detect myocardial injury. It describes the biochemistry and optimal testing timelines of commonly used markers like CK-MB, LDH, myoglobin, and cardiac troponins. It emphasizes that cardiac troponins are more specific than CK-MB for detecting myocardial damage. The document also reviews other laboratory tests that can help estimate cardiovascular disease risk, such as CRP, homocysteine, and proBNP levels.

![Although the cardiac markers are all myocardial proteins, they differ in this location within the
myocyte release after damage and clearance form the serum. Because they are markers of
myocardial damage the biochemistry of each is considered separately.
The time sequence of changes on plasma after myocardial infarction:
Markers Starts to rise
(hour)
Time after infarction of
peak elevation (hour)
Duration of rise
(days)
Ck-MB
Ck- (total)
SGOT (AST)
LD, LD-1
Myoglobin
TroponinI
4-8
4-6
6-8
12-24
1-3
4-6
24-48
24-48
24-48
48-72
6-9
24-48
2-3
3-4
4-6
7-12
1
7-14
Creatinine kinace:
CK catalyzes the formation of phosphocreatine from creatine and adenosine triphosphate.
(ATP). Several molecular forms (iso enzyme) of CK exist. They differ in their Michaels
constant and PH
optima, but the physiological significance of this is unknown. Each of the
this iso enzymes of CK is composed of two polypeptide chains.
Ck as Cardiac Marker
* Isoenzymes:
1. CK1 (CK-BB) - Brain
Smooth muscle of GIT
2. CK2 (CK-MB) – Heart (15 –40%, rest CK-MM)
[half life = 12 hr] Sk. Muscle
3. CK3 (CK-MM) -Sk. Muscle (97%, rest CK-MB)
[half life > 12 hr] Heart
Isoforms of CK-MB
1. CK-MB2 (Tissue form)
2. CK-MB1 (Serum form)
MB2/MB1 = 1.0 or < 1.0 [if > 1.5 MI]
CK Index: CK-MB x 100 [ if 4 – 25% MI]
CK
All plasma enzyme activities (including that of CK-MB) may be normal until at least four
hours after the onset of chest pain due to myocardial infarction; blood should not be taken](https://image.slidesharecdn.com/cardiacmarkers-160415204739/85/Cardiac-markers-2-320.jpg)