Continuous reporting of new cases in Spain supports the relationship between Herbalife products and liver injury. The study identified 20 cases of liver damage associated with Herbalife product use reported to the Spanish Pharmacovigilance Centers between 2003-2010. Most patients were middle-aged women, and over half required hospitalization. While most recovered, one patient developed cirrhosis. The variety of Herbalife products consumed by patients did not reveal a commonly hepatotoxic ingredient. The ongoing reports emphasize the liver safety concerns regarding Herbalife dietary supplements.





![with Herbalife products was enough to reverse the
liver damage.
When analyzing the causative agent responsible for
the hepatotoxicity related to Herbalife products, one
of the initial hypotheses considered was that some
batches could contain a certain specific hepatotoxic
agent.2,3,8
However, the facts that, since 2005, we
have information of cases occurring in different parts
of the world and that, at least in Spain, there have been
cases reported on a continuing basis suggest an actual
presence of one or more hepatotoxic components of
the Herbalife products.
In our series, all cases, but one, in which informa-
tion about liver tests was available showed hepatocel-
lular liver injury. Furthermore, nine cases (9/17, 53%)
fulfilled Hy’s law,13
which predicts a mortality not
lower than 10%. In addition, 80% of the cases were
women. The association of high bilirubin levels, hepa-
tocellular type of damage, and female sex has been
shown to be predictive of a worst outcome.14
The only patient who developed cholestatic liver
damage was also taking amoxicillin–clavulanate. This
raises the possibility that in this particular instance,
amoxicillin–clavulanate could be responsible for the
damage15
despite the chronology pointing toward
Herbalife. Nevertheless, cases of Herbalife-induced
cholestatic-mixed damage have been previously
reported.2,3
The analysis of the components of Herbalife prod-
ucts revealed the presence of two herbals previ-
ously associated with hepatotoxicity: green tea16–19
and aloe vera.20–22
These herbals are included in the
products Thermojetics, Thermo Complete, and Aloe-
Vera liquid from Herbalife. In addition, one case of
hepatitis associated with the consumption of soy,
which is a component of Formula 1 and Formula 3,
has been published.23
However, none of these herbal
medicines is included in the composition of RoseOx,
a single product that one of our patients took. Inter-
estingly, Stickel et al.8
demonstrated the hepatotoxic
effect of B. subtilis isolated from the products Formula
1, vitamin C tablets, and Herbalife specifically person-
alized, but not from RoseOx tablets. Therefore, it
is possible that the Herbalife products contain
various hepatotoxic agents, which could work alone
or by interacting with other components. Interactions
between chemicals should always be considered as
a possibility when several active ingredients are
administrated together. For example, Formula 1 from
Herbalife contains various heavy metals and carra-
geenan. Recently, Khotimchenko et al.24
have reported
carrageenans to bind heavy metals.
It is worth noting that there seems to be a general
consensus between consumers about the efficacy of
some Herbalife products to lose weight, which hap-
pens to be particularly surprising considering the
declared components of Herbalife products, such as
vitamins, minerals, and “innocuous” herbs. Moreover,
in medical literature, considerable documentation
exists related to contamination with adulterants (i.e.,
heavy metals, microorganisms, and undeclared
ingredients) in preparations formulated with herbal
medicines and marketed through several channels.25–30
To our knowledge, quality controls were not per-
formed on any of the products used by the patients in-
cluded in our series. In Spain, Herbalife products are
registered as dietary supplements in the AESAN and
are available from several Internet pages (www.
herbalife.com and others) and from local distributors,
often also consumers of Herbalife products, who sell
these products directly to their family, friends,
acquaintances, and other members of their communi-
ties. Liver injury associated with Herbalife products
has been recently reviewed by Stickel et al.31
Accord-
ing to this publication, Israel and Switzerland have not
noticed further incidents after publication of their
corresponding series, although investigators from Ice-
land and Spain continue to see new cases. In our opin-
ion, differences in the pharmacovigilance procedures or
in the batches of Herbalife products distributed in differ-
ent countries—with not exactly the same composition or
concentration of the components—could explain this
discrepancy.
The lack of knowledge of the mechanism of action in-
volved in the production of adverse reactions associated
with drugs has never been an obstacle in implementing
measures to prevent damage. Usually, these measures
are taken on the basis of clinical observations and con-
trasted epidemiological data. However, to evaluate
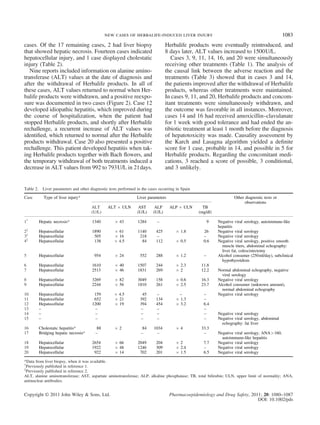
Table 6. Herbalife products taken by the patients in the published cases
Reference Total of
reported
cases
Number of patients who took it
RoseOx Other
Herbalife
products
Unknown
Schoepfer et al.,
2007 [2]
10 4 5 1
Elinav et al.,
2007 [3]
12 10 2 0
Chao et al., 2008
[7]
1 0 1 0
Stickel et al.,
2009 [8]
2 1 1 0
Jóhannsson
et al., 2010 [10]
5 5 0 0
Total 30 20 9 1
Cases from this
paper
20 9 3 8
g. manso ET AL.1086
Copyright © 2011 John Wiley & Sons, Ltd. Pharmacoepidemiology and Drug Safety, 2011; 20: 1080–1087
DOI: 10.1002/pds](https://image.slidesharecdn.com/c7c8ea138e7a-141221120659-conversion-gate02/85/Yuyos-verdes-7-320.jpg)
![efficacy and safety of herbal medicines and dietary sup-
plements is extremely difficult because they are not un-
der similar control procedures as conventional drugs
are. To know the full composition, quantitative and
qualitative, to pass strict quality controls, and to demon-
strate its effectiveness in controlled conditions are basic
and necessary requirements to be met in order to assess
the risk–benefit ratio in clinical practice.32
In conclusion, our results support the relationship
between the consumption of Herbalife products and
hepatotoxicity, underscore the concern regarding
liver-related safety of these dietary supplements—
despite the component(s) responsible for the liver
damage being unknown—and emphasize the need
to establish further regulatory measures.
ACKNOWLEDGEMENTS
We wish to express our gratitude to (i) the Spanish
Pharmacovigilance System for allowing us to analyze
and publish the FEDRA data and (ii) those health
professionals whose adverse event reports were sub-
mitted to the Spanish Pharmacovigilance System.
On behalf of the Spanish Group for the Study of Drug-
Induced Liver Disease, we would like to thank the
clinical group in each case recruitment center: Hospital
Universitario Virgen de la Victoria, Málaga (coordinat-
ing center): RJ Andrade, MI Lucena, C Stephens, Y
Borraz, M García-Cortés, E Ulzurrun, M Robles-Díaz,
and I Moreno; Hospital Costa del Sol, Málaga: JM
Navarro; Xeral-Calde Hospital, Lugo: S Avila-Nasi;
Carlos Haya Hospital, Málaga: M Jiménez and R
González-Grande; Hospital Puerto Real, Cádiz: JM
Pérez-Moreno; and Hospital Infanta Cristina, Badajoz:
JL Montero.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
KEY POINTS
• Previous cases occurred in several countries have
linked HerbalifeW
products and hepatotoxicity.
• Since 2003–2010, at least 20 cases of liver injury
related to these dietary supplements occurred in
Spain. Two of them showed positive rechallenge.
• Our results underscore the concern about these
adverse reactions and emphasize the need to es-
tablish further regulatory measures.
REFERENCES
1. Hoffmann M, Marbet UA, Hurni A, Bianchi L, Goldie H. Rezidiv medikamentös-
toxischen einer Hepatitis. Schweiz Med Forum 2005; 5: 147–8.
2. Schoepfer AM, Engel A, Fattinger K, et al. Herbal does not mean innocuous: ten
cases of severe hepatotoxicity associated with dietary supplements from Herba-
life products. J Hepatol 2007; 47: 521–6.
3. Elinav E, Pinsker G, Safadi R, et al. Association between consumption of Herba-
life nutritional supplements and acute hepatotoxicity. J Hepatol 2007; 47:
514–20.
4. Stickel F. Slimming at all costs: Herbalife-induced liver injury. J Hepatol 2007;
47: 444–6.
5. Duque JM, Ferreiro J, Salgueiro E, Manso G. Hepatotoxicity associated with
the consumption of herbal slimming products. Med Clin (Barc) 2007; 128:
238–9.
6. Manso G, López-Rivas L, Duque JM, Salgueiro E. Spanish reports of hepa-
totoxicity associated with Herbalife products. J Hepatol 2008; 49:
289–90.
7. Chao S, Anders M, Turbay M, Olaiz E, Mc Cormack L, Mastai R. Toxic hepatitis
by consumption Herbalife products: a case report. Acta Gastroenterol Latinoam
2008; 38: 274–7.
8. Stickel F, Droz S, Patsenker E, Bögli-Stuber K, Aebi B, Leib SL. Severe hepato-
toxicity following ingestion of Herbalife nutritional supplements contaminated
with Bacillus subtilis. J Hepatol 2009; 50: 111–7.
9. Seeff LB. Are herbals as safe as their advocates believe?. J Hepatol 2009; 50:
13–6.
10. Jóhannsson M, Ormarsdóttir S, Olafsson S. Hepatotoxicity associated with the
use of Herbalife. Laeknabladid 2010; 96: 167–72.
11. Benichou C. Criteria of drug-induced liver disorders. J Hepatol 1990; 11: 272–6.
12. Web page of Herbalife http://www.herbalife.com [accessed 27 June 2011].
13. Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol
Drug Saf 2006; 15: 241–3.
14. Andrade RJ, Lucena MI, Fernández MC, et al. Drug-induced liver injury: an
analysis of 461 incidences submitted to the Spanish registry over a 10-year pe-
riod. Gastroenterology 2005; 129: 512–21.
15. Lucena MI, Andrade RJ, Fernández MC, et al. Determinants of liver expression
of amoxicillin–clavulanate hepatotoxicity: a prospective series from Spain.
Hepatology 2006; 44: 850–6.
16. Pedrós C, Cereza G, Garcia N, Laporte JR. Liver toxicity of Camellia sinensis
dried extract etanolic. Med Clin (Barc) 2003; 121: 598–9.
17. Bonkovsky HL. Hepatotoxicity associated with supplements contain-
ing Chinese green tea (Camellia sinensis). Ann Intern Med 2006; 144:
68–71.
18. García-Cortés M, Borraz Y, Lucena MI, et al. Liver injury induced by “natural
remedies”: an analysis of cases submitted to the Spanish Liver Toxicity Registry.
Rev Esp Enferm Dig 2008; 100: 688–95.
19. Sarma DN, Barrett ML, Chavez ML, et al. Safety of green tea extracts: a system-
atic review by the U.S. pharmacopeia. Drug Saf 2008; 31: 469–84.
20. Rabe C, Musch A, Schirmacher P, Kruis W, Hoffmann R. Acute hepatitis in-
duced by an aloe vera preparation: a case report. World J Gastroenterol 2005;
11: 303–4.
21. Kanat O, Ozet A, Ataergin S. Aloe vera-induced acute toxic hepatitis in a healthy
young man. Eur J Intern Med 2006; 17: 589.
22. Bottenberg MM, Wall GC, Harvey RL, Habib S. Oral aloe vera–induced hepati-
tis. Ann Pharmacother 2007; 41: 1740–3.
23. Borghi-Scoazec G, Vial T, Bobin JY, Trepo C. Phytosya(R)-induced cytolytic
hepatitis. Gastroenterol Clin Biol 2002; 26: 181–3.
24. Kotimchenko YS, Khozhaenko EV, Khotimchenko MY, Kolenchenko EA,
Kovalev VV. Carrageenans as a source of new drugs with metal binding proper-
ties. Drugs 2010; 8: 1106–21
25. Ernst E. Toxic heavy metals and undeclared drugs in Asian herbal medicines.
Trend Pharmacol Sci 2002; 23: 136–9.
26. Stedmann C. Herbal hepatotoxicity. Semin Liver Dis 2002; 22: 195–206.
27. De Smet PA. Health risks of herbal remedies: an update. Clin Pharmacol Ther
2004; 76: 1–17.
28. Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol 2005; 43:
901–10.
29. Seef LB. Herbal hepatotoxicity. Clin Liver Dis 2007; 11: 577–96.
30. Chan TY. Potential risks associated with the use of herbal anti-obesity products.
Drug Saf 2009; 32: 453–6.
31. Stickel F, Kessebohm K, Weimann R, Seitz HK. Review of liver injury associ-
ated with dietary supplements. Liver Int 2011; 31: 595–605.
32. Cohen PA. American roulette—contaminated dietary supplements. N Engl J Med
2009; 361: 1523–5.
new cases of herbalife-induced liver injury 1087
Copyright © 2011 John Wiley & Sons, Ltd. Pharmacoepidemiology and Drug Safety, 2011; 20: 1080–1087
DOI: 10.1002/pds](https://image.slidesharecdn.com/c7c8ea138e7a-141221120659-conversion-gate02/85/Yuyos-verdes-8-320.jpg)