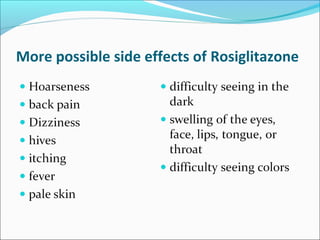
Here are the missing words for the abstract:
1. because
2. statistically
3. meta-
4. while
5. Conducting
6. during
Under-reporting of cardiovascular events in the rofecoxib Alzheimer disease studies.
Abstract
Background; In September 2004, rofecoxib (Vioxx) was removed from the market because it was found to produce a statistically doubling of cardiovascularthrombotic (CVT) events in a placebo-controlled study. Its manufacturer stated that this was the first clear evidence of such risk and criticized meta- analyses of earlier CVT risk for focusing on investigator-reported events. We studied contemporaneously adjudicated CVT events to assess the information







![True or False [see paragraph number]
1.[3] There was international consensus that Tamiflu helped protect the
patient from more severe forms of flu
2. [4] The claims made about Tamiflu were based on a series of meta-analyses
3.[5] There are contradictions in the FDA’s response to claims about the
effectiveness of Tamiflu
4.[6] It is more important for regulators to assess clinical study reports than
journal publications of clinical trials
5.[7] Analysis of the Tamiflu clinical study report has given radical new
information about Avandia, Neurontin and Vioxx
6.[9] To ensure reliable evidence synthesis will require a change in the present
situation regarding manufacturers’ confidentiality
7.[10] The manufacturers give regulators access to all their trial reports when
a new drug is being approved
8.[11] It is not easy to gain access from the FDA to reviews, memos and other
correspondence](https://image.slidesharecdn.com/tamifluandclinicalstudyreports-130115040153-phpapp01/85/Tamiflu-and-clinical-study-reports-8-320.jpg)





![Under-reporting of cardiovascular events in the rofecoxib Alzheimer disease
studies.
Abstract
Background; In September 2004, rofecoxib (Vioxx) was removed from the market
-----1----- it was found to produce a -----2----- doubling of cardiovascularthrombotic (CVT)
events in a placebo-controlled study. Its manufacturer stated that this was the first clear
evidence of such risk and criticized -----3----- analyses of earlier CVT risk for focusing on
investigator-reported events. We studied contemporaneously adjudicated CVT events to
assess the information on cardiovascular risk available -----4----- the drug was in
widespread use.
Methods: -----5----- an intention-to-treat analysis of adjudicated CVT deaths, we analyzed
detailed patient-level data collected -----6----- 3 randomized placebo-controlled trials
of rofecoxib versus placebo that had been designed to define the drug's possible role in
the prevention or treatment of Alzheimer disease. All trials -----7----- completed by April
2003.
Results: In the 3 studies combined, the data indicated that rofecoxib -----8----- tripled
the risk of confirmed CVT death (risk ratio = 3.57 [1.48-9.72], P = .004). This finding
reached the P < .05 level of significance by June 2001.
Conclusion: Intention-to-treat analysis of placebo-controlled studies of rofecoxib for
Alzheimer disease demonstrated that the drug produced a significant increase in
confirmed CVT deaths nearly 40 months before it was removed from the market.-----
9-----, published analyses of these trials -----10----- restricted to on-treatment analyses
(ending 14 days after cessation of treatment) that did not reveal this risk. Intention-to-
treat analyses of clinical trial data can reveal important information about potential drug
risks and -----11----- performed routinely and reported in a -----12----- manner.](https://image.slidesharecdn.com/tamifluandclinicalstudyreports-130115040153-phpapp01/85/Tamiflu-and-clinical-study-reports-17-320.jpg)




![1.[2] Strong sales are a good indication that a drug is safe F
2.[3] There was international consensus that Tamiflu helped protect the
patient from more severe forms of flu T
3.[4]The claims made about Tamiflu were based on a series of meta-analyses F
4.[5] There are contradictions in the FDA’s response to claims about the
effectiveness of Tamiflu T
5.[6] It is more important for regulators to assess clinical study reports than
journal publications of clinical trials T
6.[7] Analysis of the Tamiflu clinical study report has given radical new
information about Avandia, Neurontin and Vioxx F
7.[9] To ensure reliable evidence synthesis will require a change in the present
situation regarding manufacturers’ confidentiality T
8.[10] The manufacturers give regulators access to all their trial reports when
a new drug is being approved F
9.[11] It is not easy to gain access from the FDA to reviews, memos and other
correspondence T
10.[12] Roche have not kept their promise to give reviewers access to
information T](https://image.slidesharecdn.com/tamifluandclinicalstudyreports-130115040153-phpapp01/85/Tamiflu-and-clinical-study-reports-22-320.jpg)


![Am Heart J. 2012 Aug;164(2):186-93. Epub 2012 Jul 3.
Under-reporting of cardiovascular events in the rofecoxib Alzheimer disease studies.
Madigan D, Sigelman DW, Mayer JW, Furberg CD, Avorn J.
Abstract
BACKGROUND:
In September 2004, rofecoxib (Vioxx) was removed from the market after it was found to produce a
near doubling of cardiovascularthrombotic (CVT) events in a placebo-controlled study. Its
manufacturer stated that this was the first clear evidence of such risk and criticized previous
analyses of earlier CVT risk for focusing on investigator-reported events. We studied
contemporaneously adjudicated CVT events to assess the information
on cardiovascular risk available while the drug was in widespread use.
METHODS:
Using an intention-to-treat analysis of adjudicated CVT deaths, we analyzed detailed patient-level
data collected during 3 randomized placebo-controlled trials of rofecoxib versus placebo that had
been designed to define the drug's possible role in the prevention or treatment of Alzheimer disease.
All trials had been completed by April 2003.
RESULTS:
In the 3 studies combined, the data indicated that rofecoxib more than tripled the risk of confirmed
CVT death (risk ratio = 3.57 [1.48-9.72], P = .004). This finding reached the P < .05 level of
significance by June 2001.
CONCLUSION:
Intention-to-treat analysis of placebo-controlled studies of rofecoxib for Alzheimer disease
demonstrated that the drug produced a significant increase in confirmed CVT deaths nearly 40
months before it was removed from the market. By contrast, published analyses of these trials were
restricted to on-treatment analyses (ending 14 days after cessation of treatment) that did not reveal
this risk. Intention-to-treat analyses of clinical trial data can reveal important information about
potential drug risks and should be performed routinely and reported in a timely manner.](https://image.slidesharecdn.com/tamifluandclinicalstudyreports-130115040153-phpapp01/85/Tamiflu-and-clinical-study-reports-25-320.jpg)