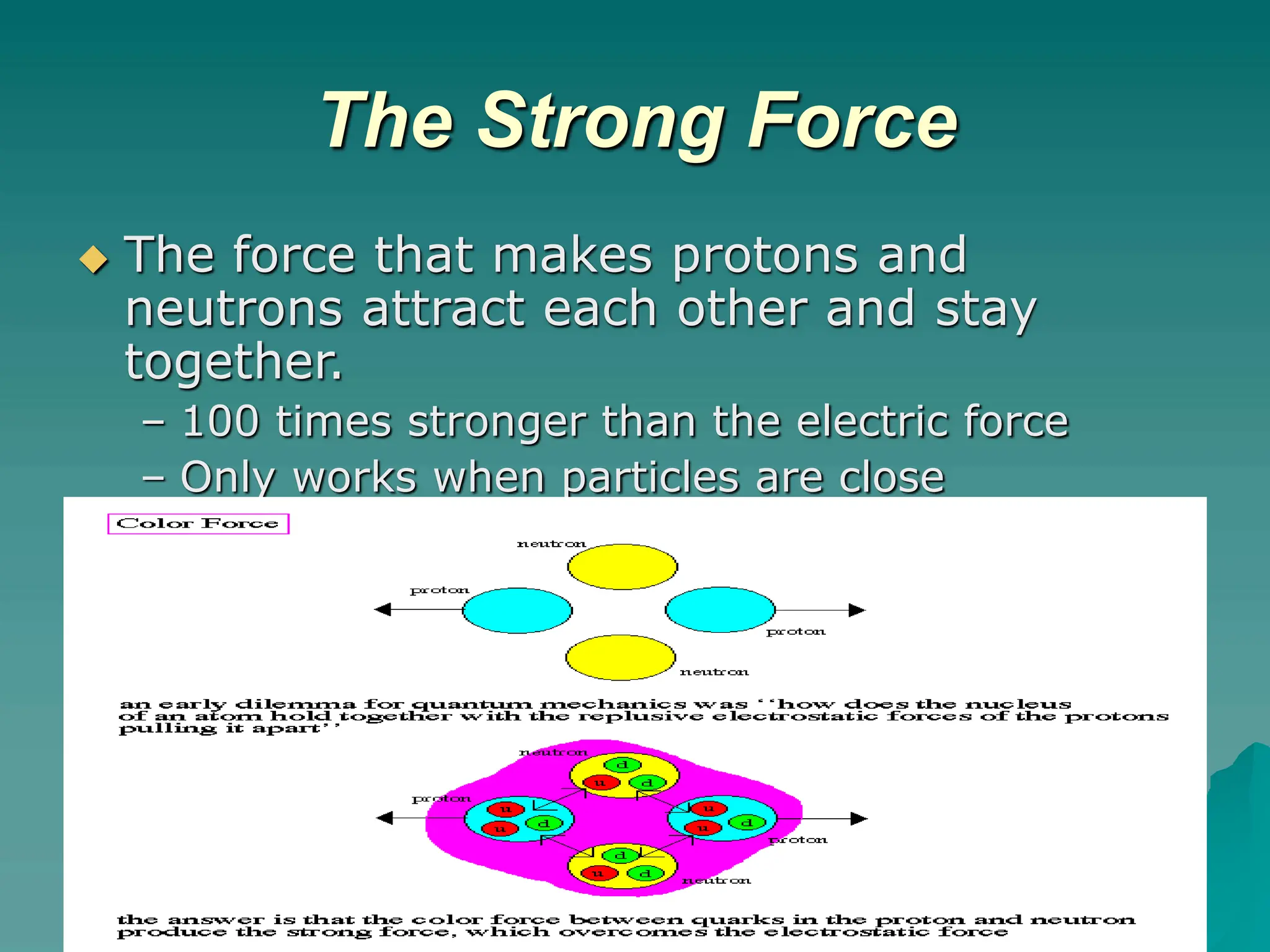
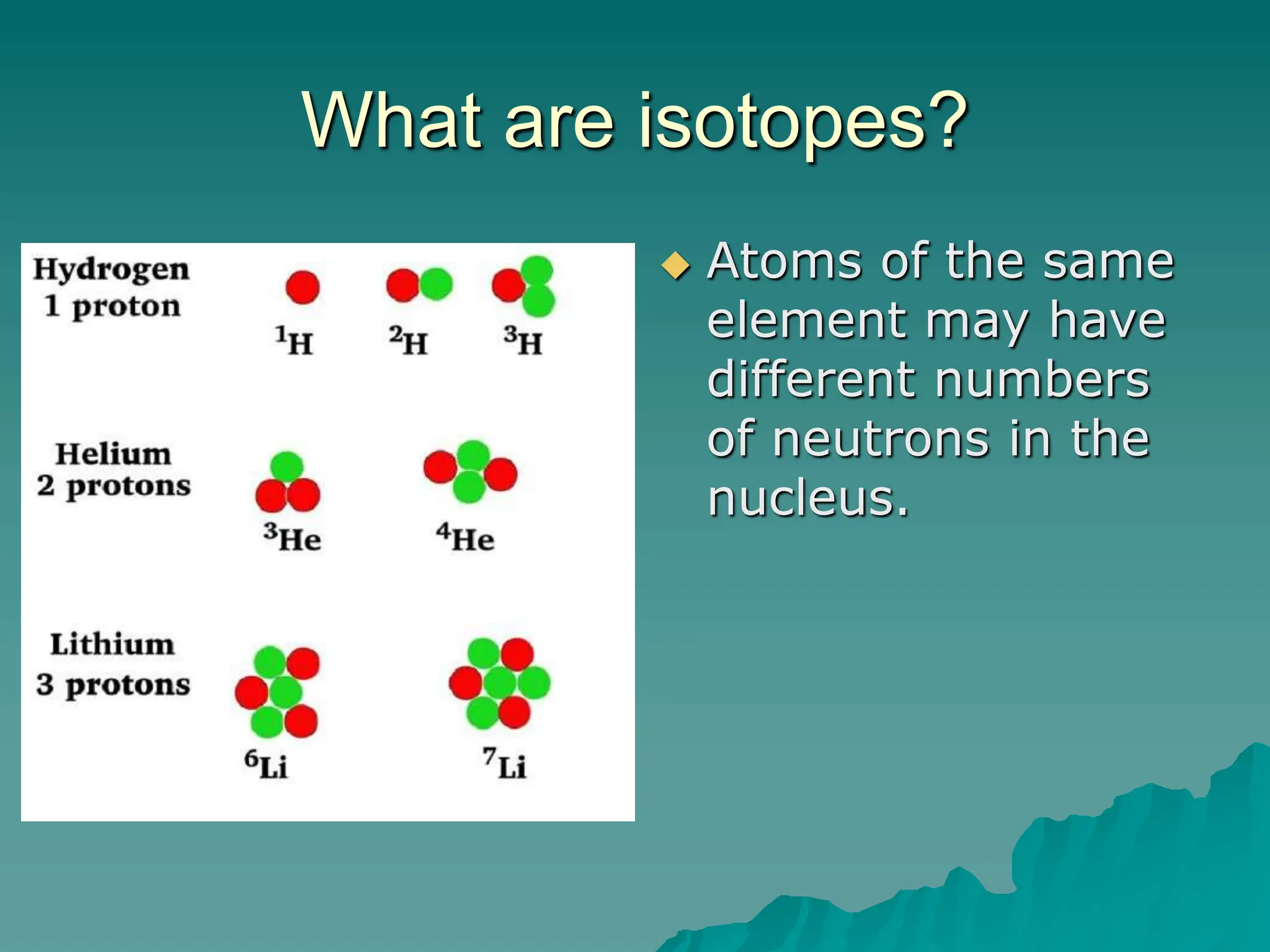
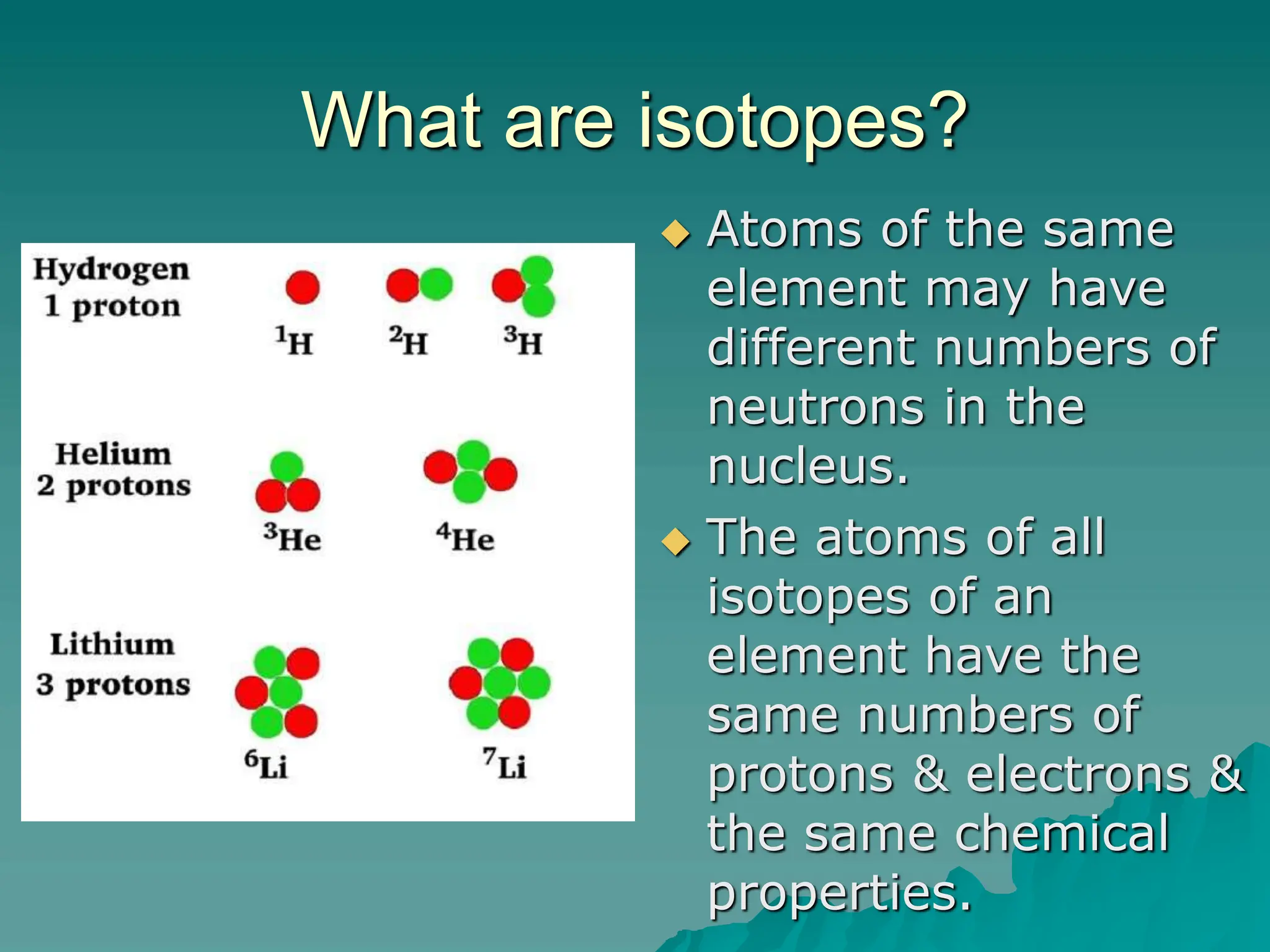
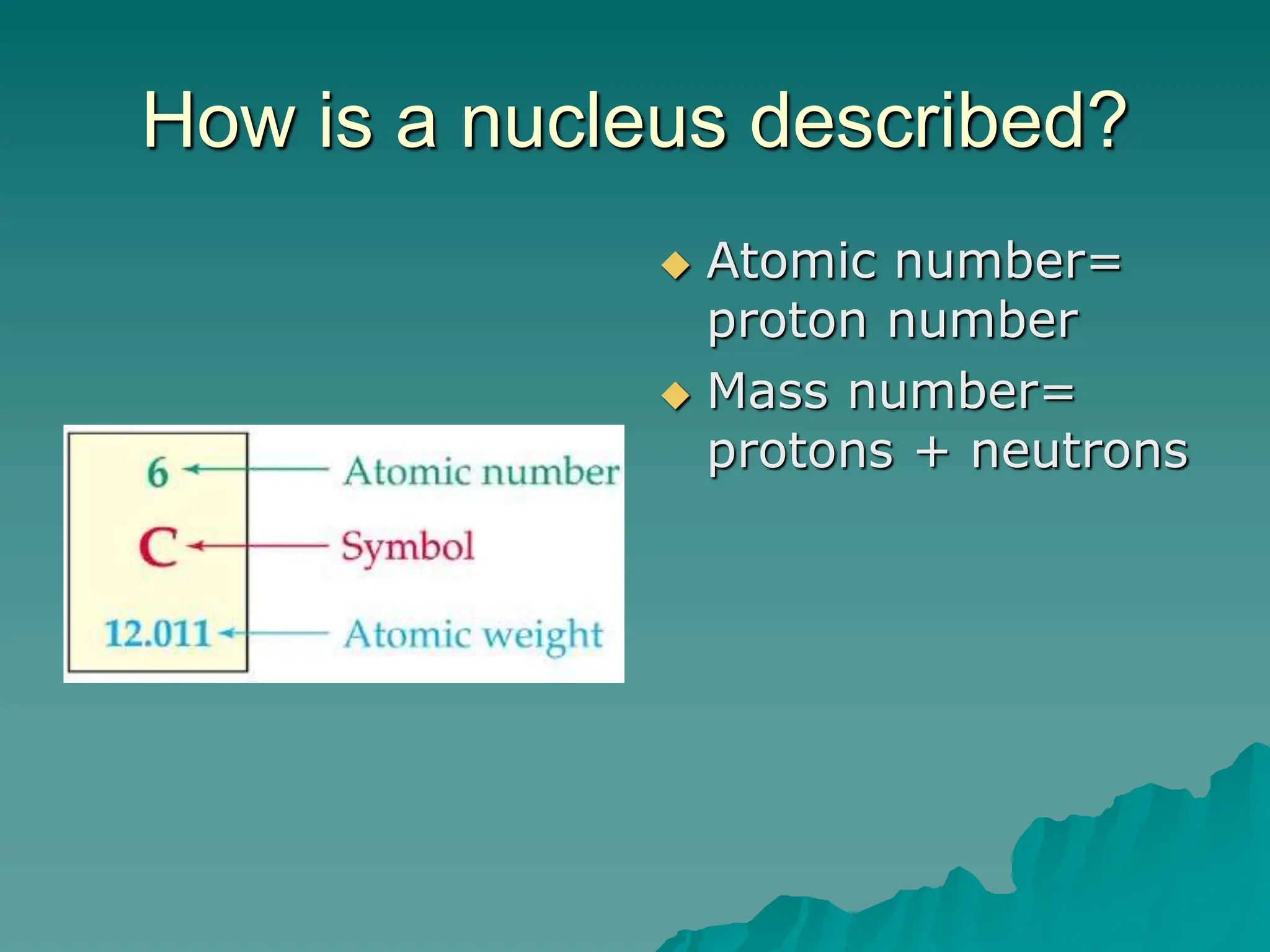
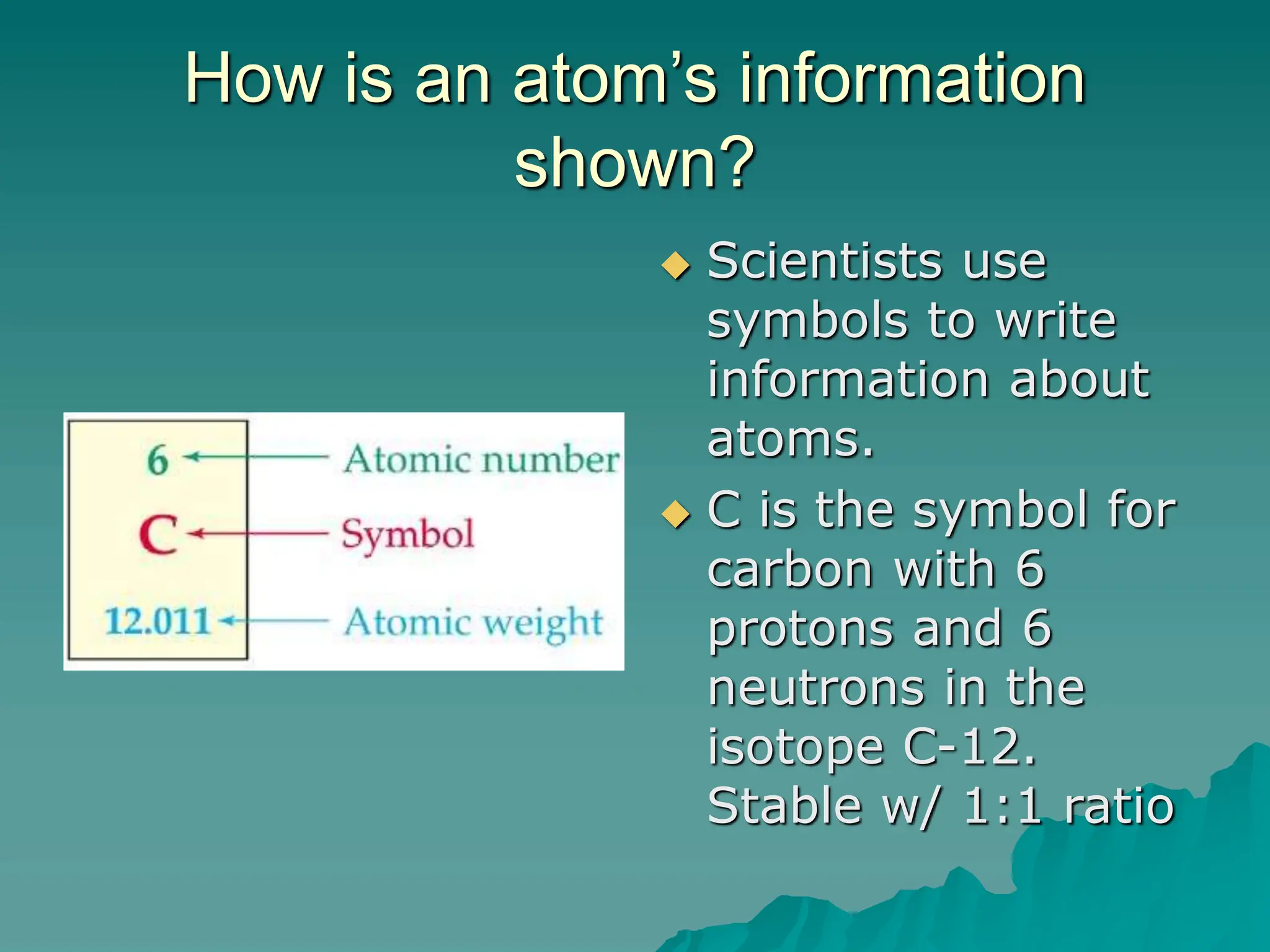
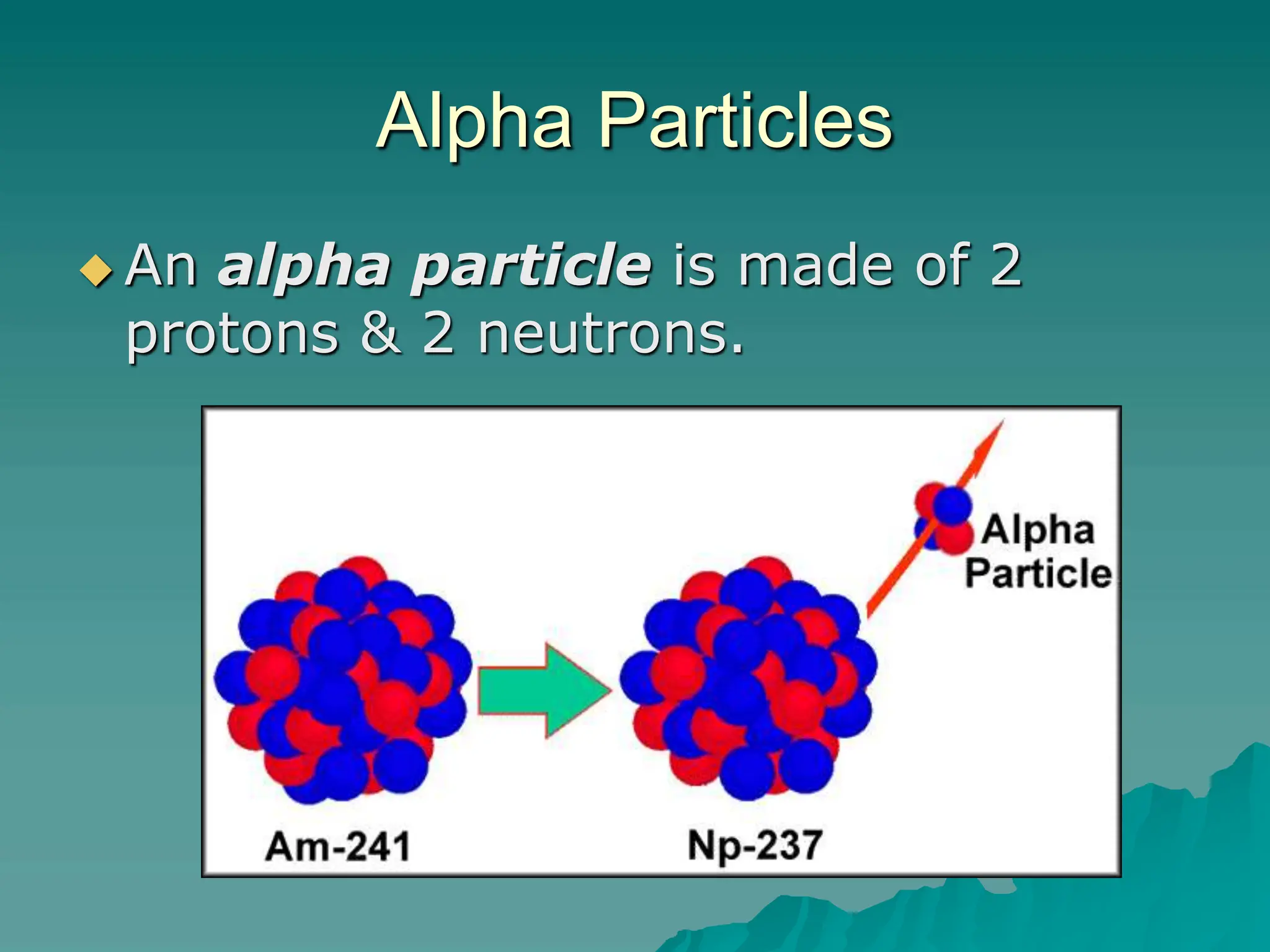
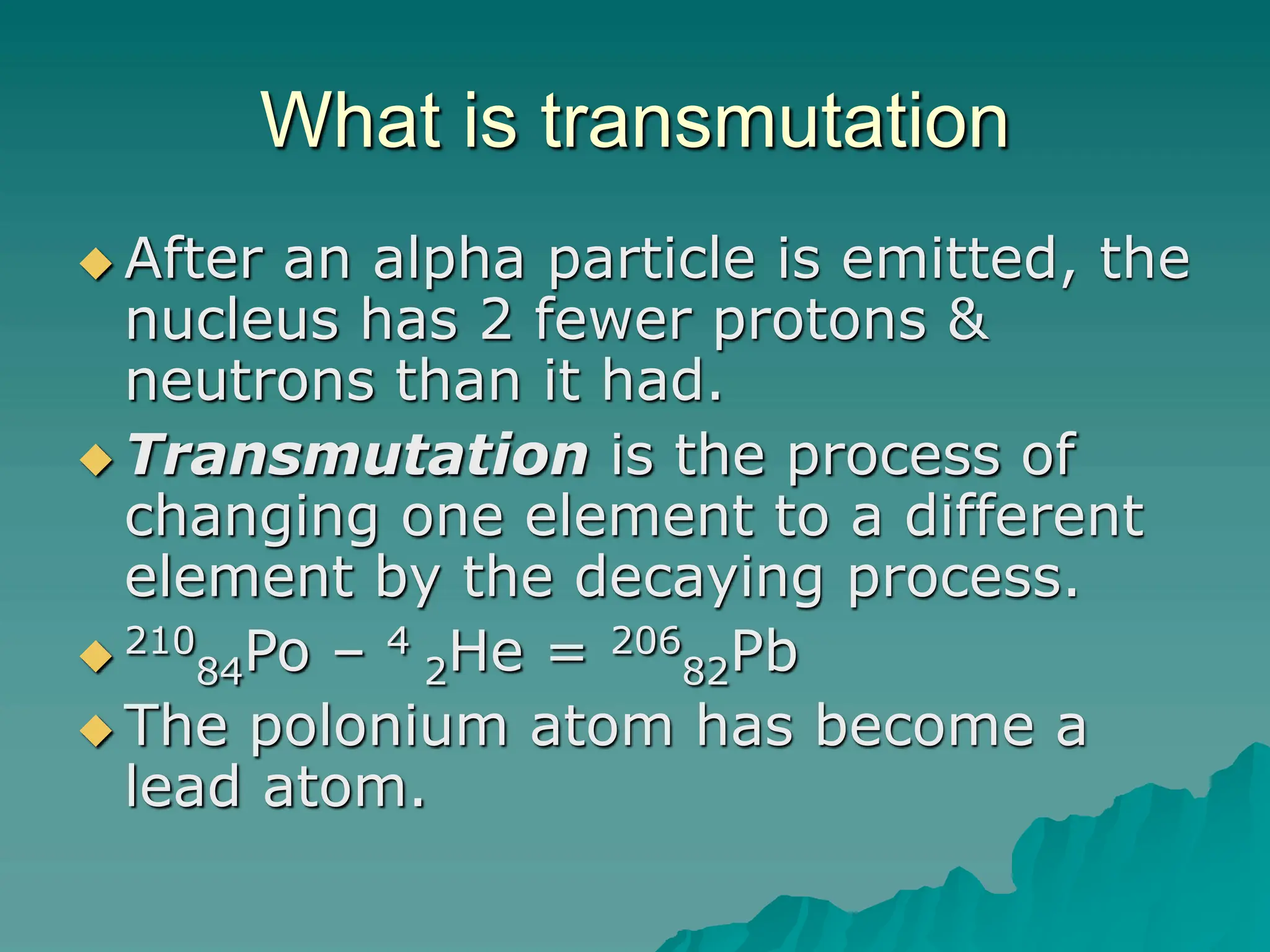
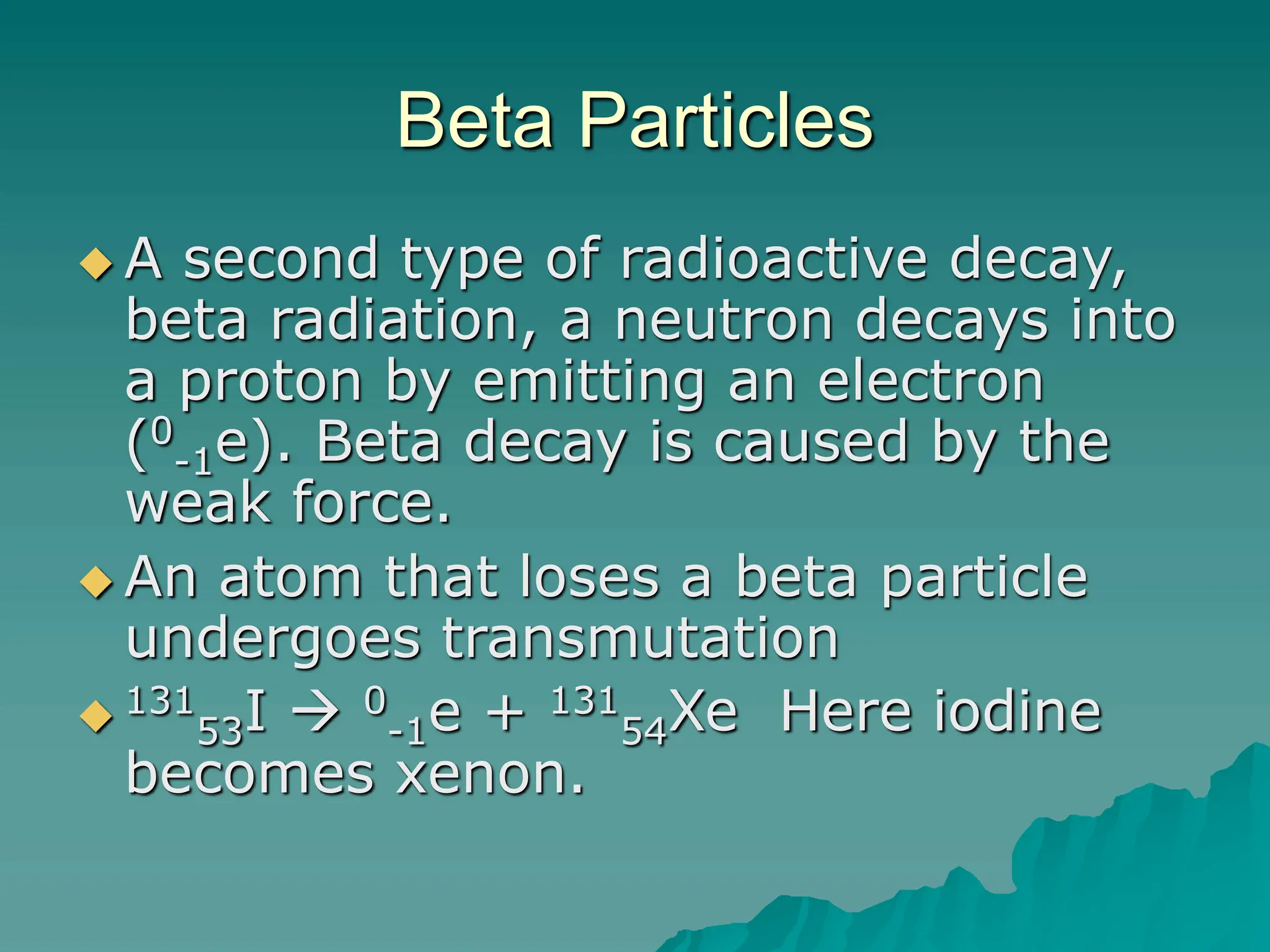
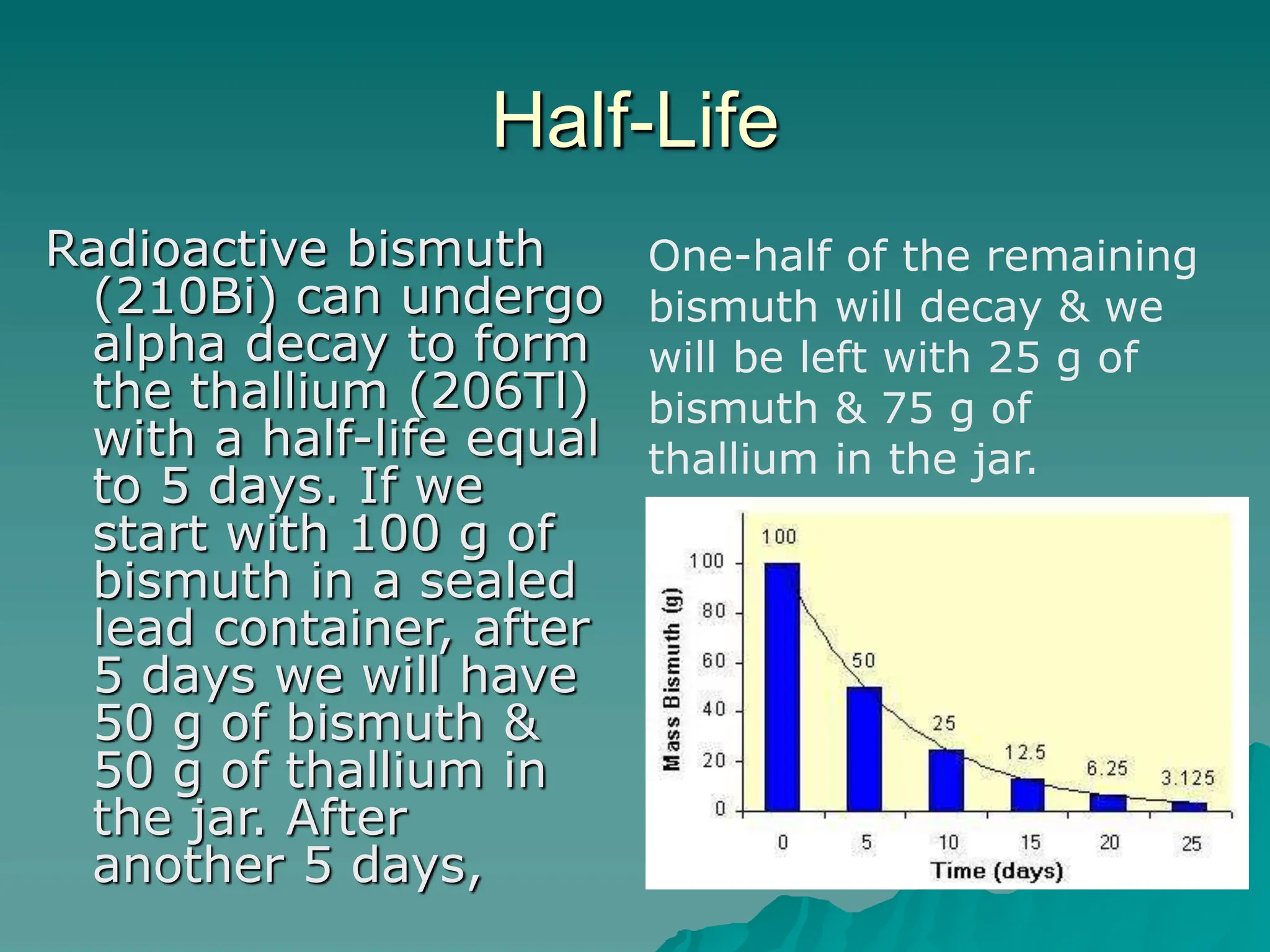
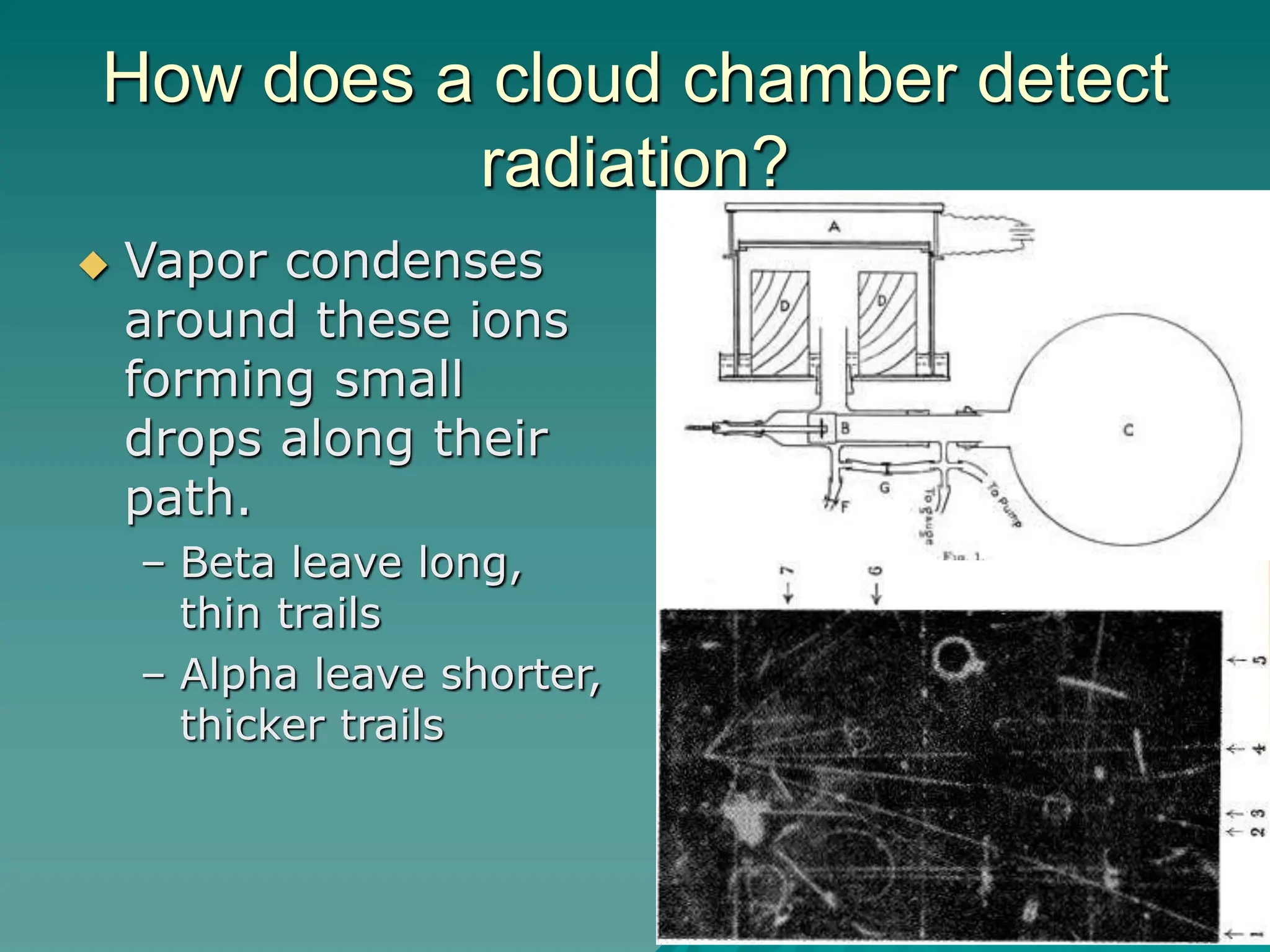
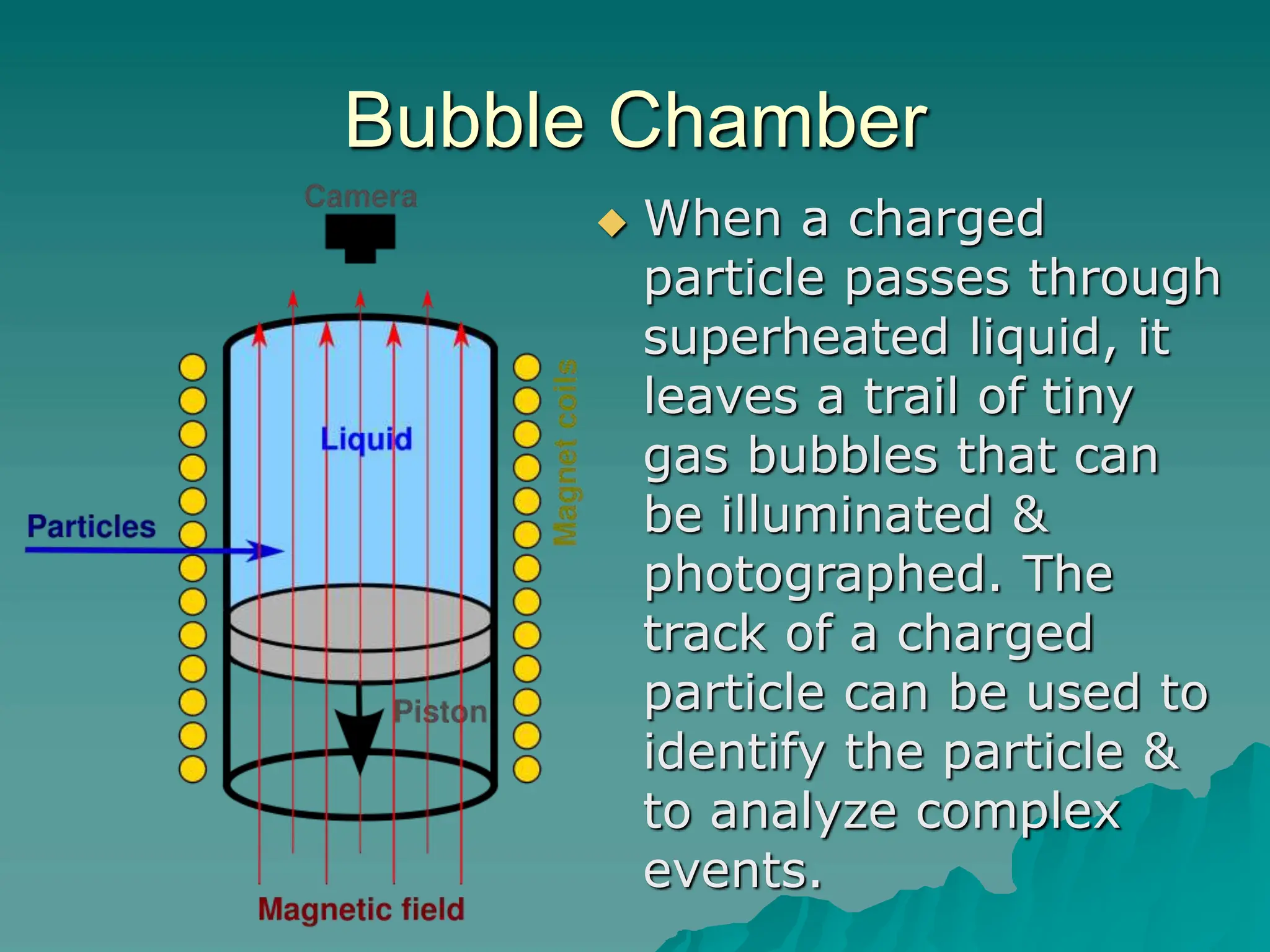
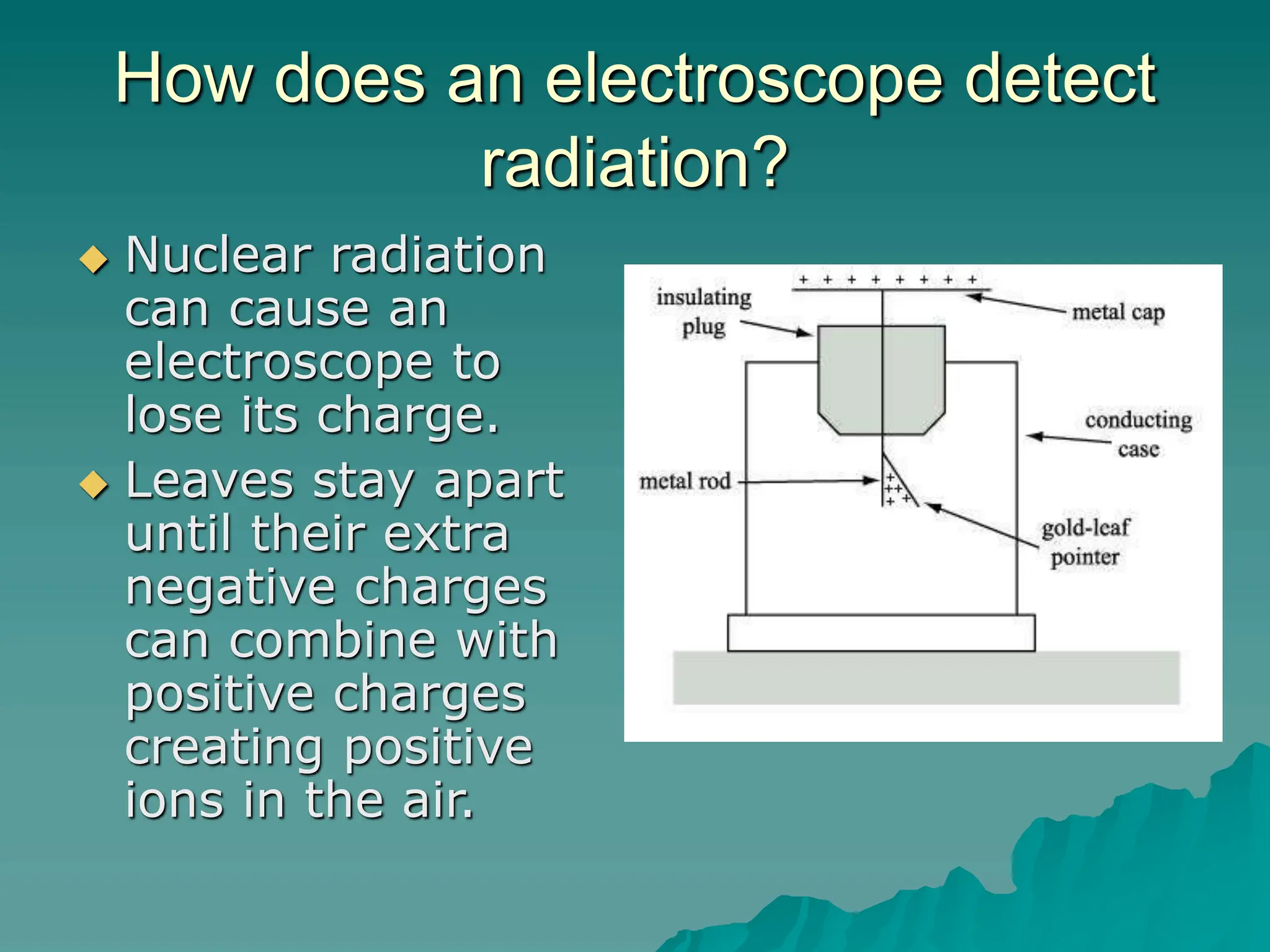
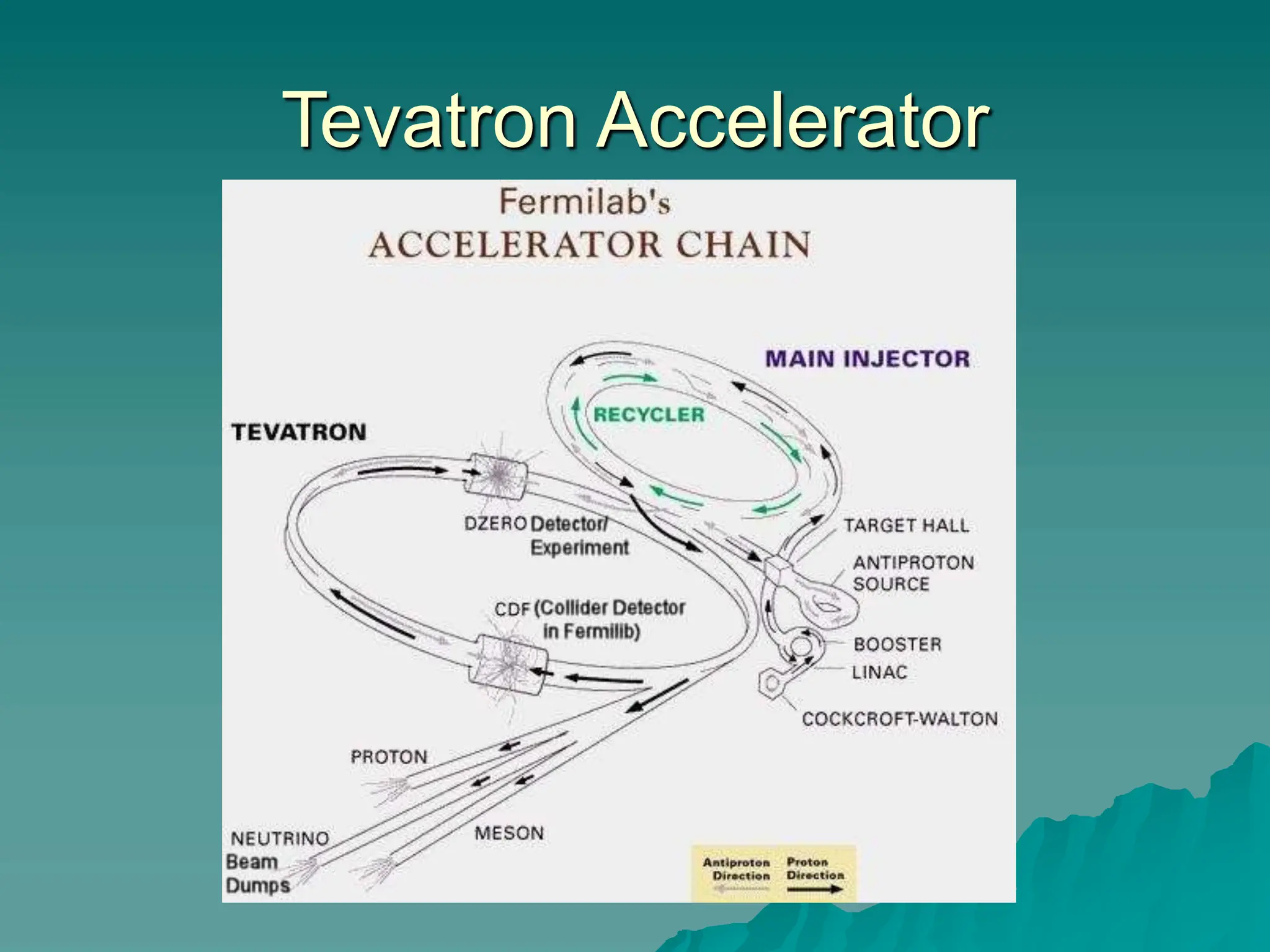
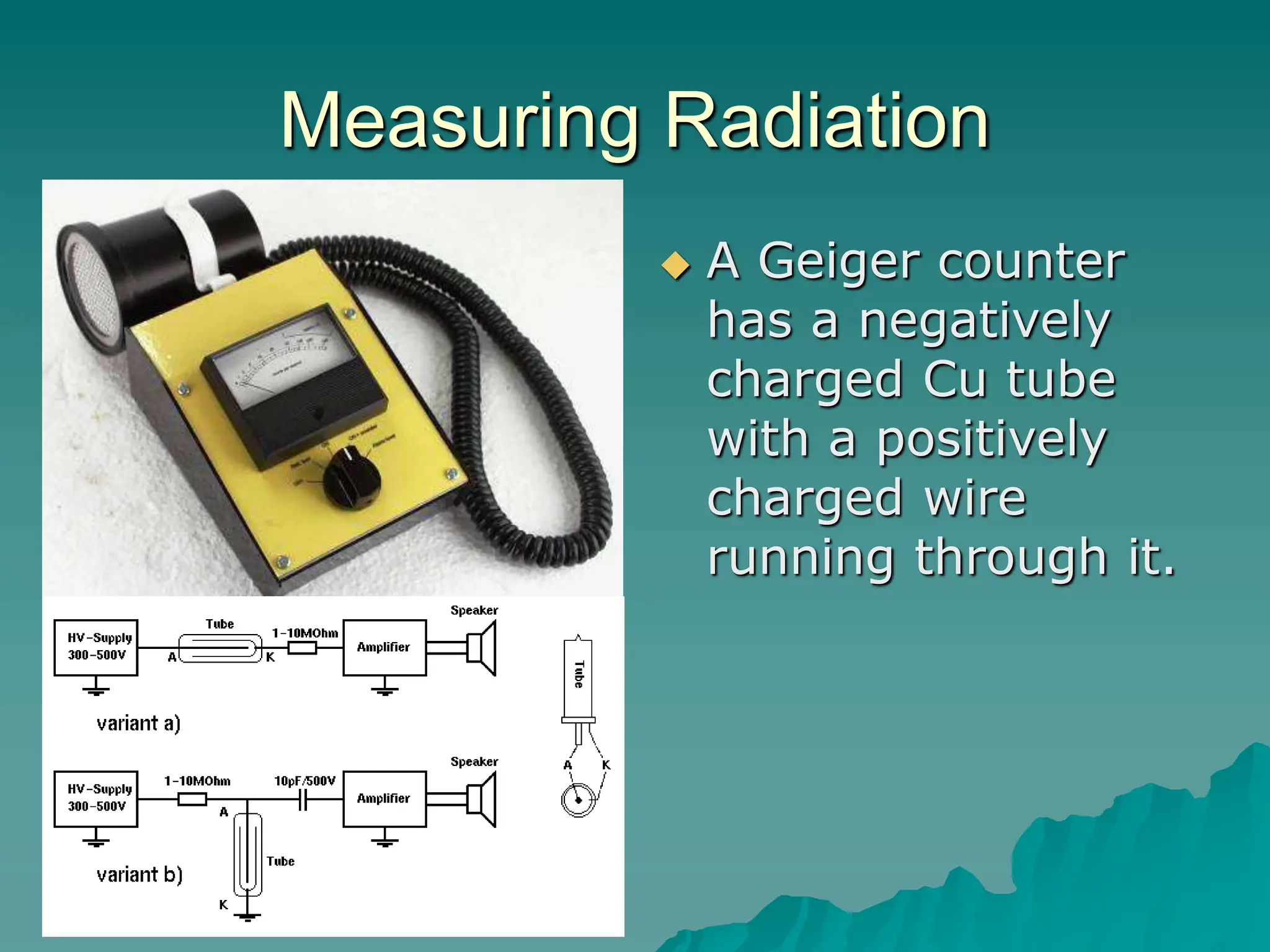
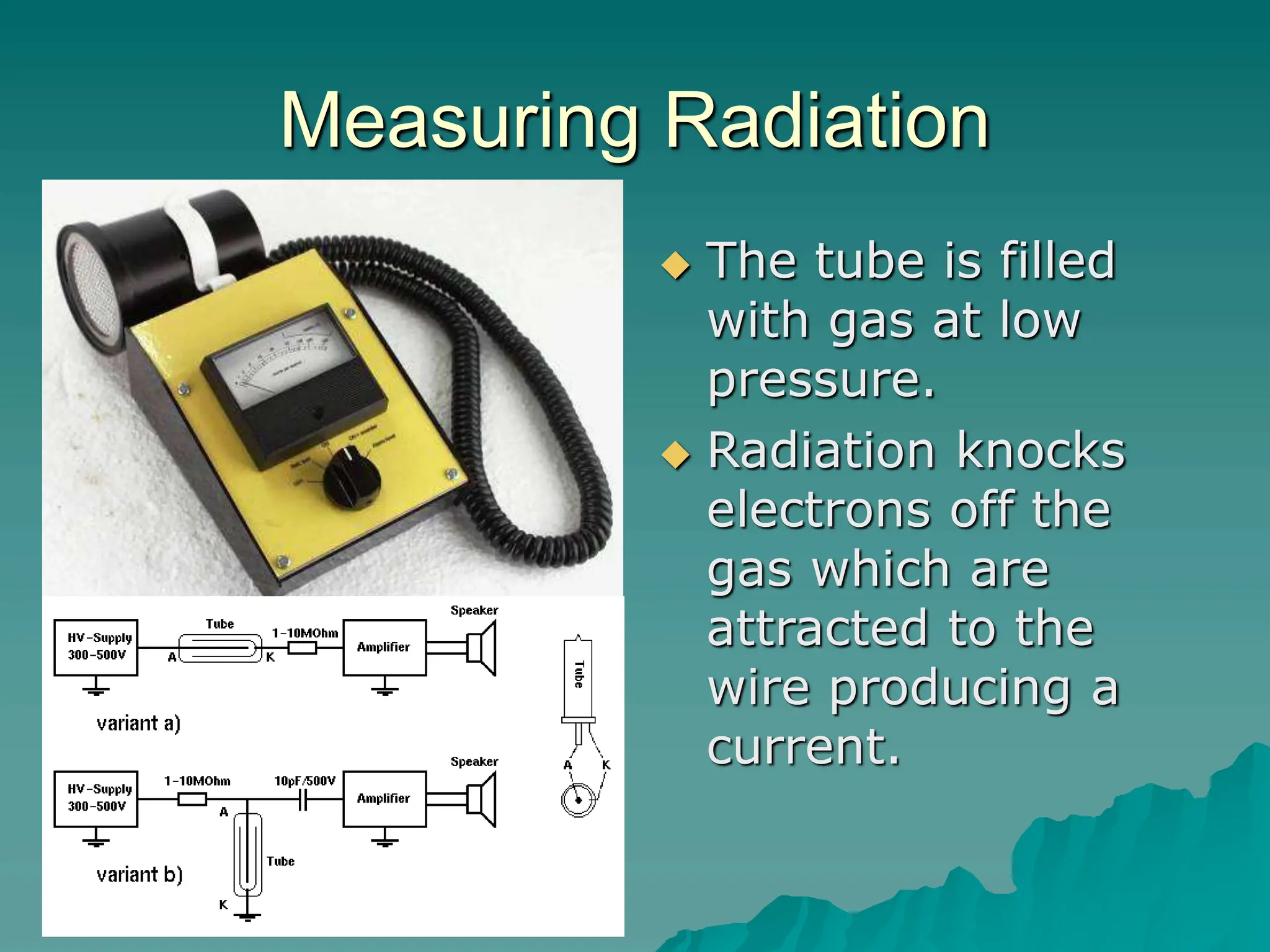
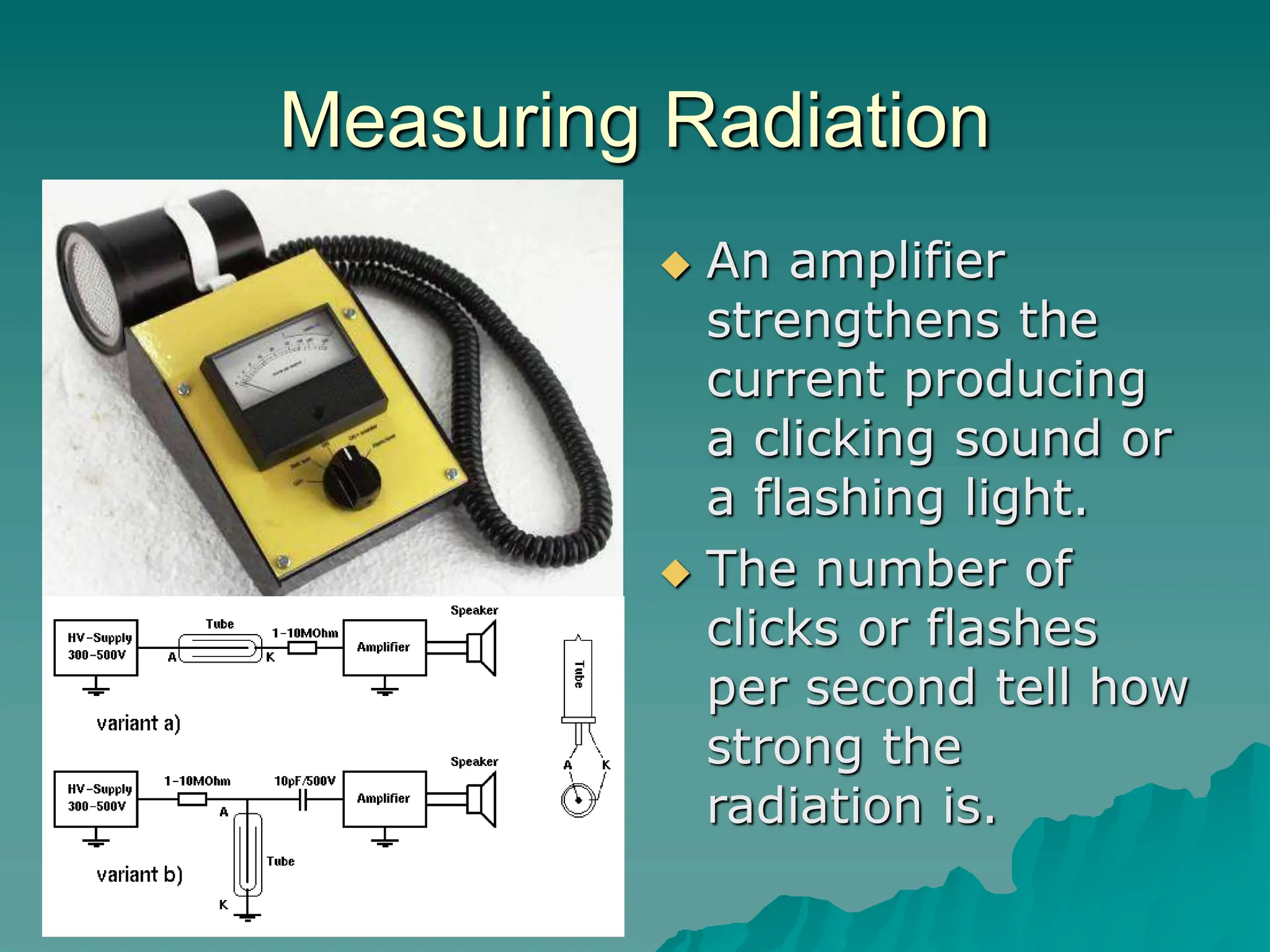
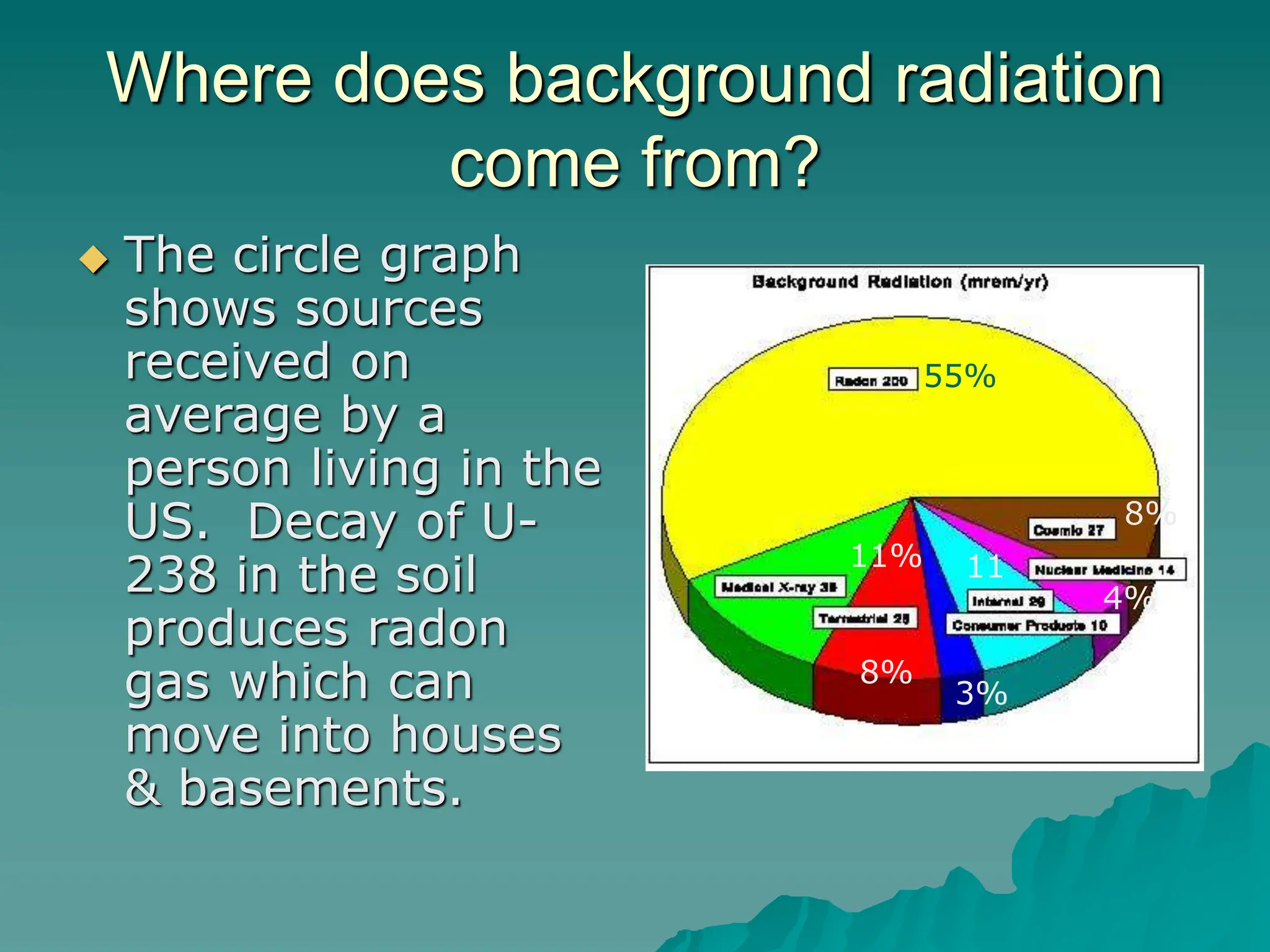
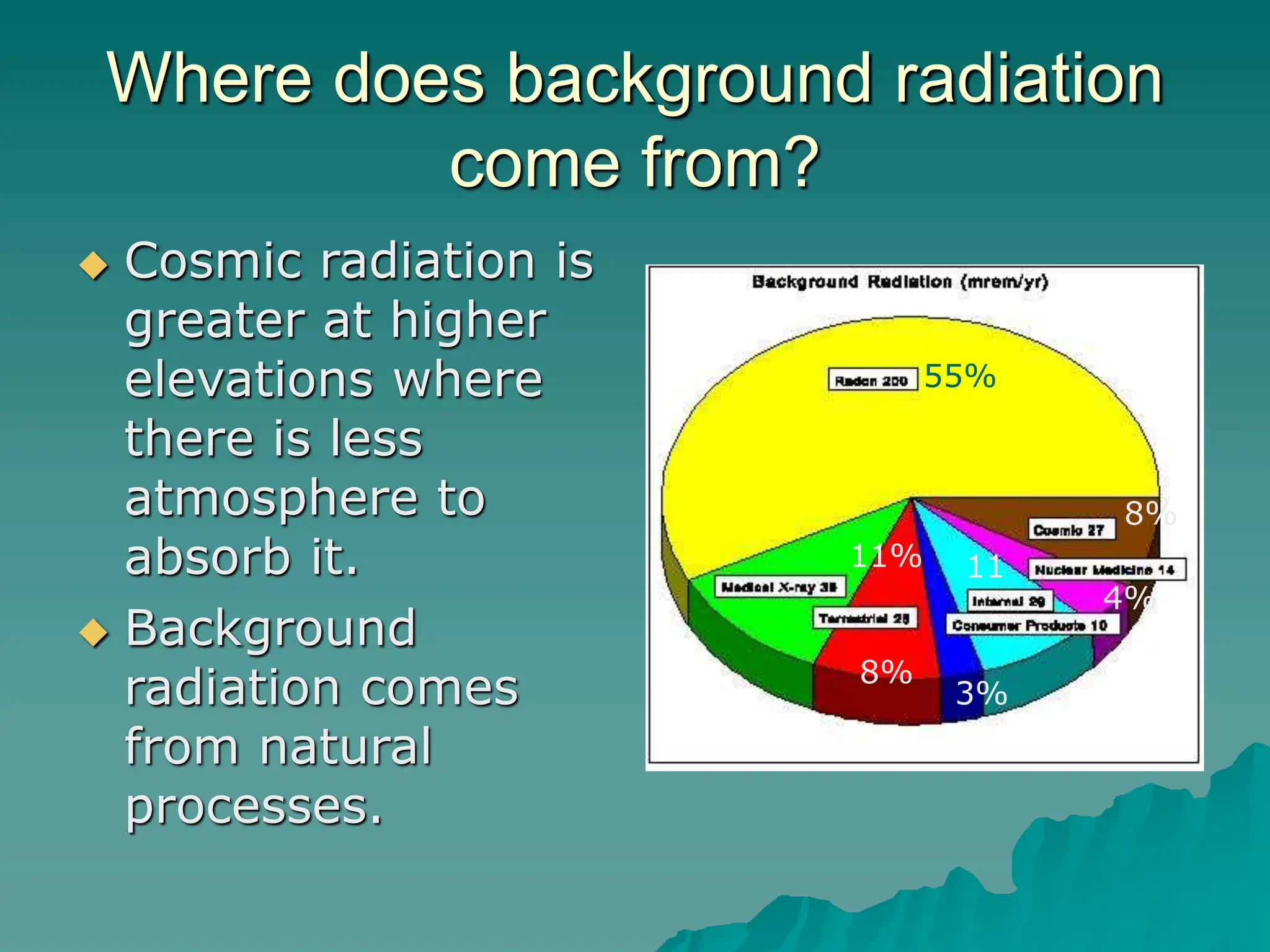
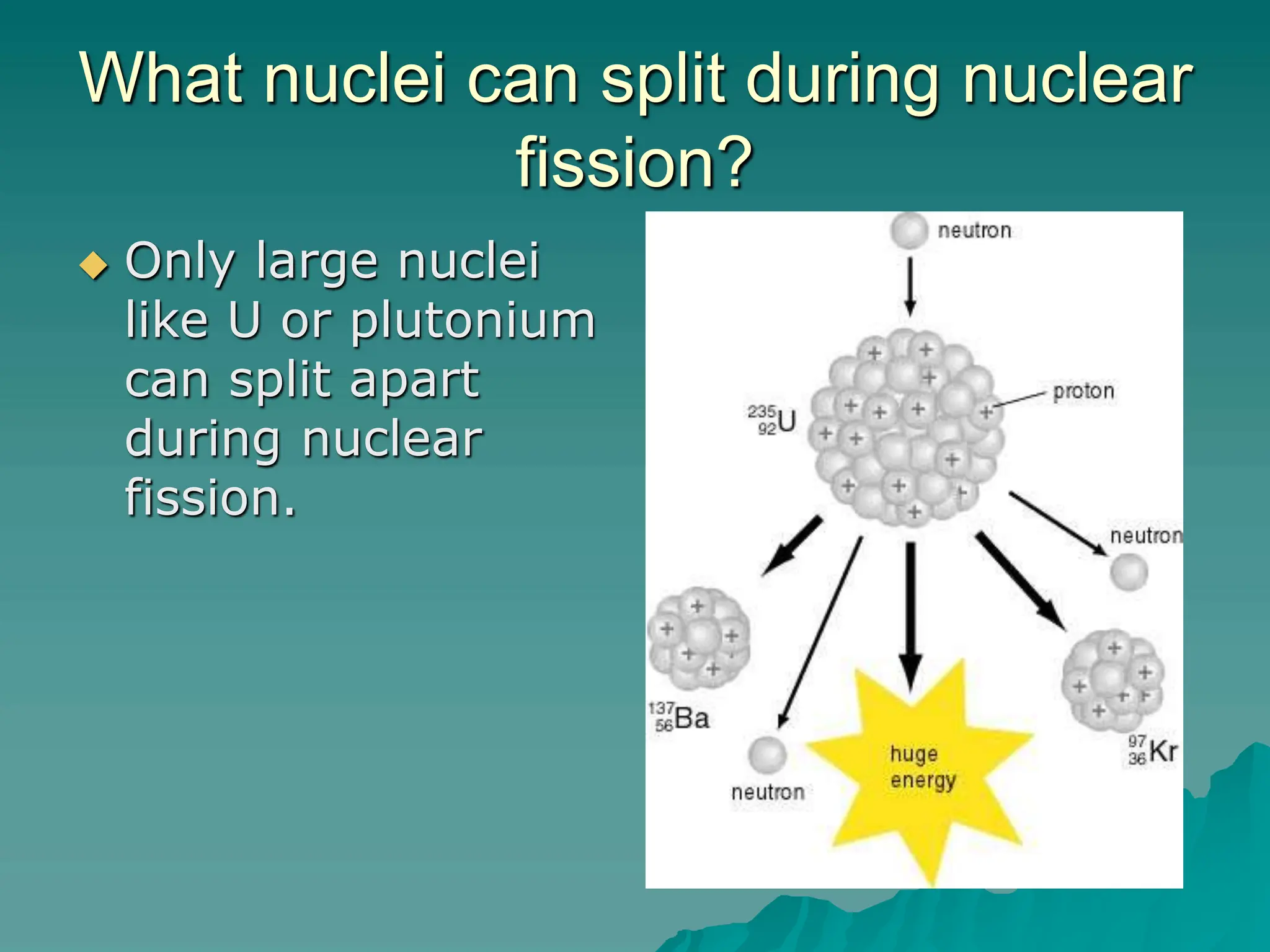
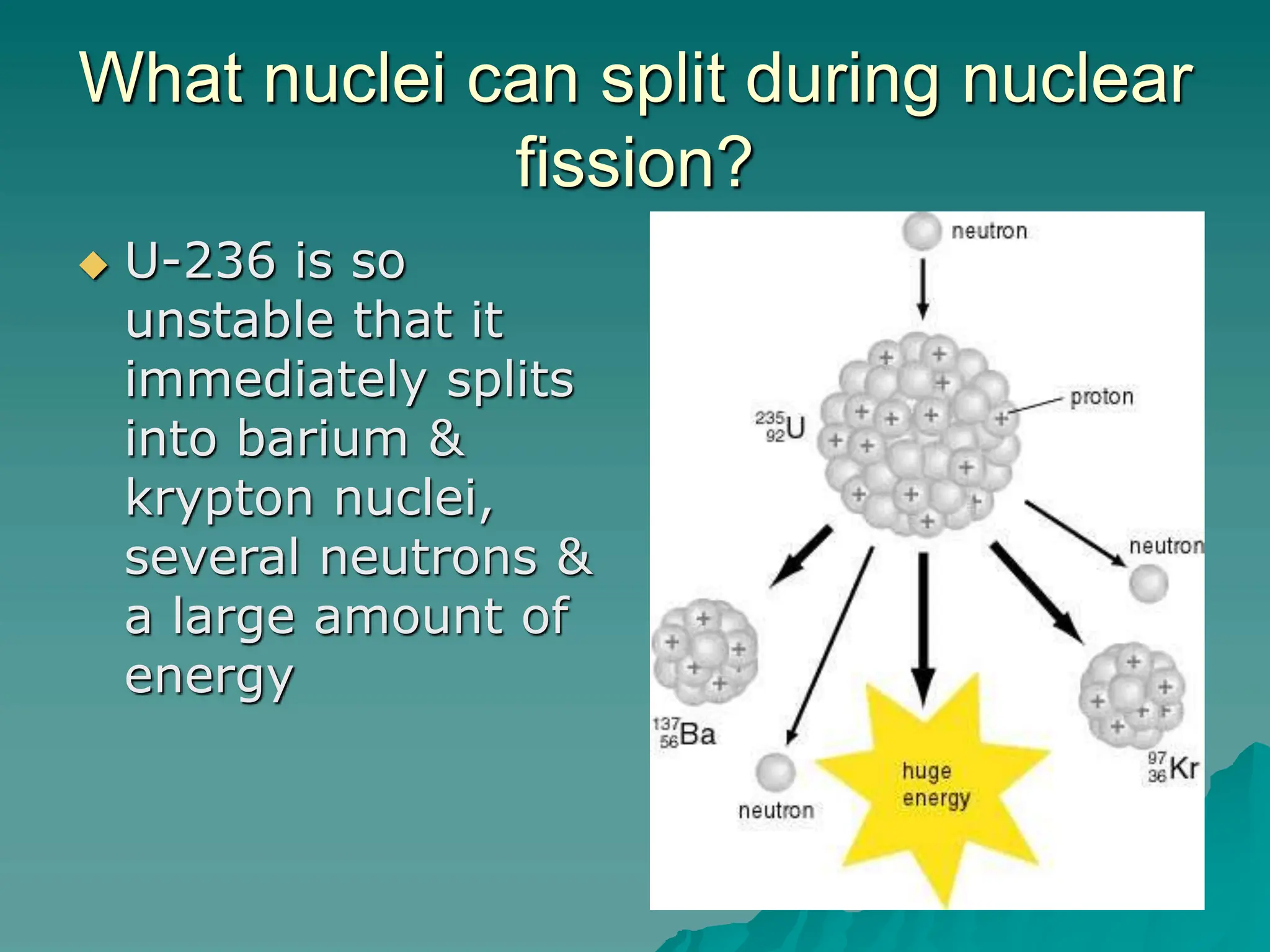
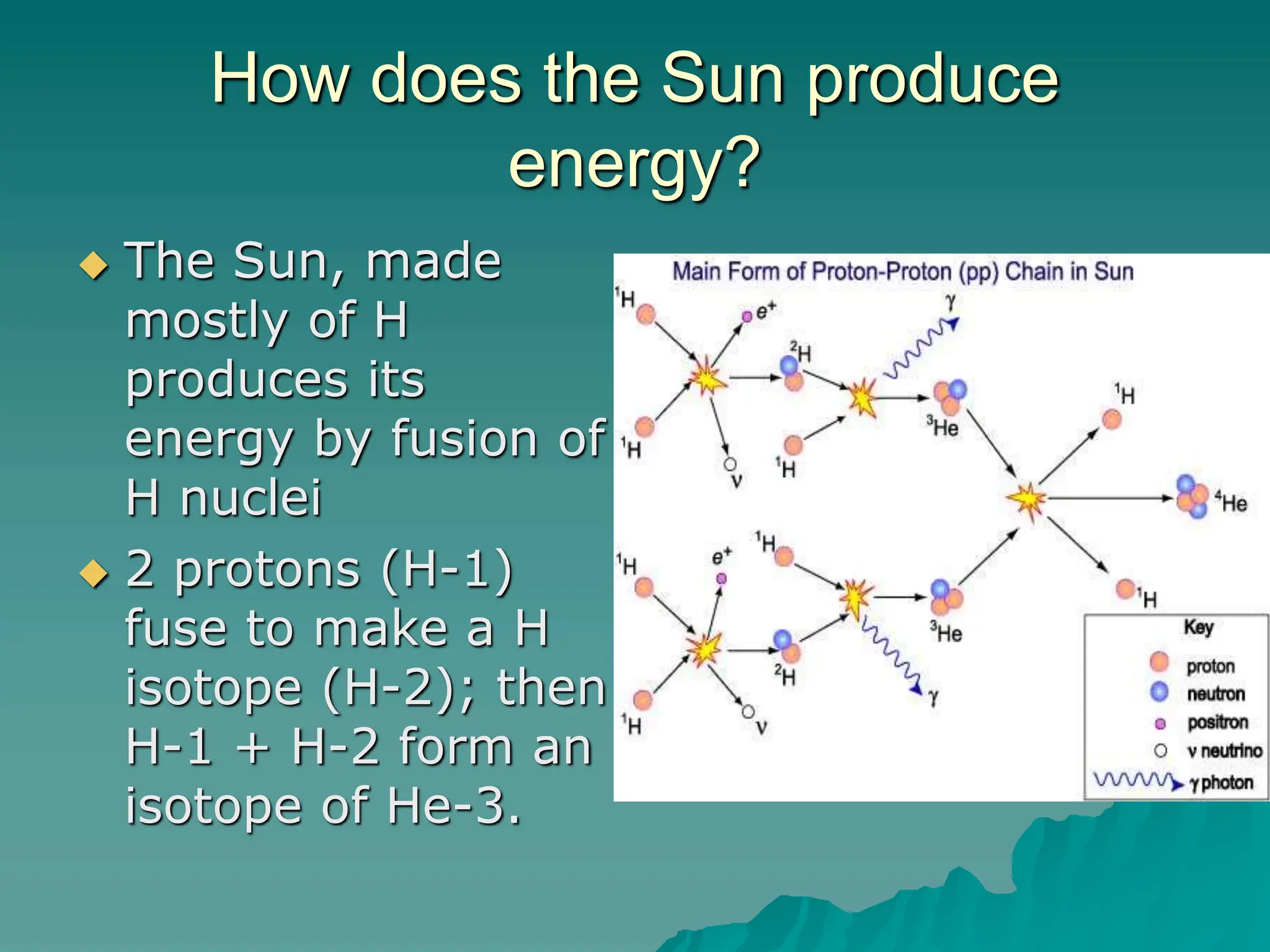
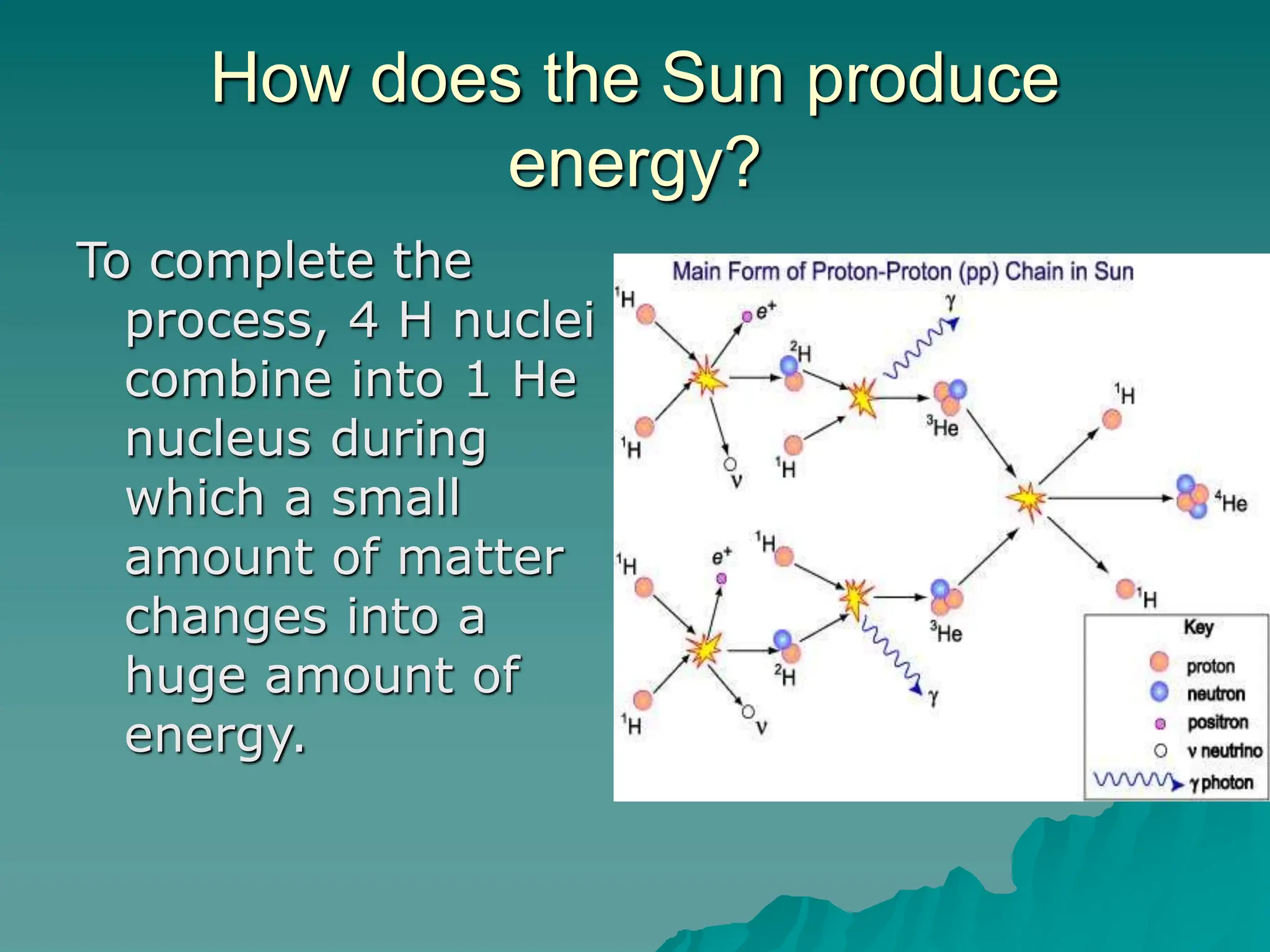
This document provides an overview of radioactivity and nuclear reactions. It discusses the structure of the atom and nucleus, the three types of nuclear radiation (alpha, beta, gamma), radioactive decay, half-life, and methods for detecting radioactivity like cloud chambers, bubble chambers, electroscopes, and Geiger counters. Radioactive dating methods that use isotopes like carbon-14 and uranium are also summarized.