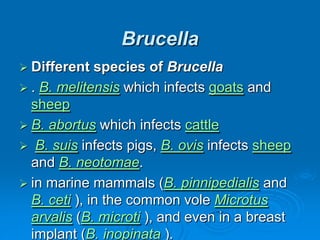
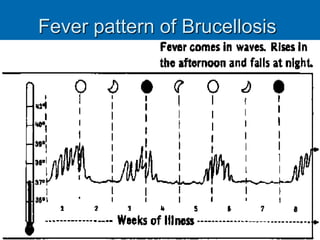
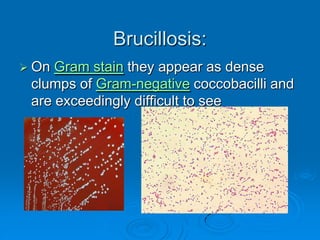
Brucella is a genus of Gram-negative bacteria that causes brucellosis. It is transmitted through contact with infected animals or ingestion of contaminated animal products. Different Brucella species infect various animals like cattle, goats, pigs, and marine mammals. Symptoms include fever, sweating, joint and muscle pain. Diagnosis involves blood culture, antibody tests, or biopsy showing granulomas. Treatment requires combination antibiotic therapy for several weeks due to the intracellular nature of the bacteria. Prevention involves pasteurizing milk and vaccinating animals.