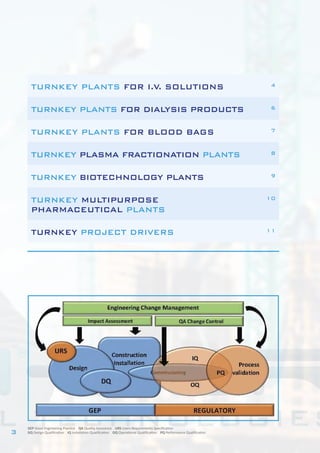
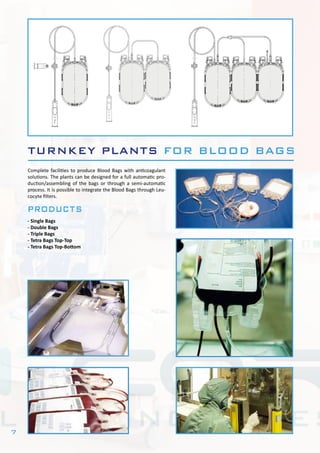
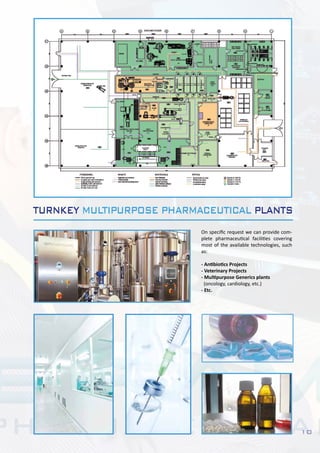
This document describes Bram-Cor's turnkey pharmaceutical projects. Bram-Cor specializes in designing, engineering, constructing, and validating complete new pharmaceutical facilities. Their services include procuring licenses and know-how transfers, conceptual and detailed engineering, validation planning, equipment installation and testing, training, and regulatory support. They have capabilities across many drug product types including intravenous solutions, dialysis products, blood bags, plasma fractionation, biotechnology, and multipurpose facilities. Bram-Cor aims to provide clients with reliable technologies and ensure their projects meet all applicable regulatory and quality requirements.