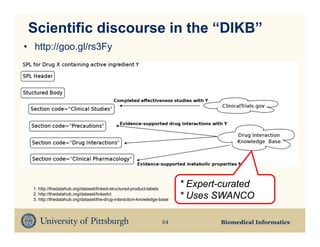
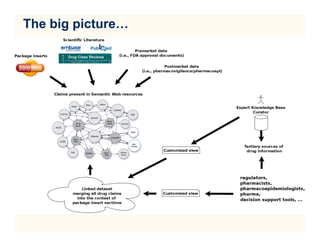
The document discusses the need for updating drug package inserts due to missing information on drug interactions and metabolic properties, particularly highlighting the limitations of current labels. It proposes using semantic web technologies and natural language processing to create a linked open data store that enhances medication safety information in these inserts. A proof of concept is presented, demonstrating how linked datasets can be integrated into package labels to provide updated and comprehensive drug safety information.



![Missing drug-drug interactions
• Example:
– “Both clopidogrel and ticlopidine significantly inhibited the
CYP2B6-catalyzed bupropion hydroxylation. Patients
receiving either clopidogrel or ticlopidine are likely to require
dose adjustments when treated with drugs primarily
metabolized by CYP2B6.” [1]
• Search bupropion drug package inserts for
“clopidogrel”
– No mention at all in Aplenzin ER insert [2]
– Mention in the generic tablet insert [3], but refers only
to hypothetical interaction
1. Turpeinen M, Tolonen A, Uusitalo J, Jalonen J, Pelkonen O, Laine K. Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by
bupropion hydroxylation. Clin Pharmacol Ther. 2005 Jun;77(6):553-9.
2. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2315d6e5-144d-4163-9711-f855c759cf8e
3. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=e537ed50-6677-45eb-9e62-af62d3831f19
4 Biomedical Informatics](https://image.slidesharecdn.com/boyce-cshals-2012-linked-spls-final-120228072423-phpapp02/85/Boyce-cshals-2012-linked-sp-ls-final-4-320.jpg)
![Missing metabolic properties
• Cimetidine inhibition of CYP3A4 and CYP1A2
metabolism
– Noted in an FDA draft guidance [1] but not PI for the
generic tablet [2]
• Fluconazole inhibition of CYP3A4 metabolism
– Noted in an FDA draft guidance [1] but not directly in
the fluconazole solution PI [3]
• Inferable from the stated interaction with midazolam
• See others like this in [4]
1. FDA. FDA Guideline: Drug Interaction Studies – Study Design, Data Analysis, and Implications for Dosing and Labeling. Rockville, MD: Food and Drug
Administration; 2006. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072101.pdf.
2. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=3a2f773d-b67f-4e83-b8d7-fce6b2887efe
3. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=26672a97-adf3-9f1d-4bdc-b2606747bbf5
4. Boyce R, Harkema H, Conway M. Leveraging the semantic web and natural language processing to enhance drug-mechanism knowledge in drug product labels.
In: Proceedings of the 1st ACM International Health Informatics Symposium. IHI ’10. New York, NY, USA: ACM; 2010:492–496. Available at:
http://doi.acm.org/10.1145/1882992.1883070.
5 Biomedical Informatics](https://image.slidesharecdn.com/boyce-cshals-2012-linked-spls-final-120228072423-phpapp02/85/Boyce-cshals-2012-linked-sp-ls-final-5-320.jpg)
![Missing age-related clearance data
• The PI for citalopram [1] notes age-related
pharmacokinetic changes
– “…subjects ≥ 60 years of age were compared to younger
subjects in two normal volunteer studies. In a single-dose study,
citalopram AUC and half-life were increased in the elderly
subjects by 30% and 50%, respectively”
• However, clearance (Cl) would be the preferred measure
of age-related change – is there literature on this?
– Yes! [2-4]
• The package inserts rarely have this information even
when published [5]
1. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4259d9b1-de34-43a4-85a8-41dd214e9177
2. Reis M, Lundmark J, Bengtsson F. Therapeutic drug monitoring of racemic citalopram: a 5-year experience in Sweden, 1992-1997. Ther Drug Monit. 2003;25(2):183-
191.
3. Gutierrez M, Abramowitz W. Steady-state pharmacokinetics of citalopram in young and elderly subjects. Pharmacotherapy. 2000;20(12):1441-1447.
4. Bies RR, Feng Y, Lotrich FE, et al. Utility of sparse concentration sampling for citalopram in elderly clinical trial subjects. J Clin Pharmacol. 2004;44(12):1352-1359.
5. Boyce RD, Handler SM, Karp JF, Hanlon JT. Age-Related Changes in Antidepressant Pharmacokinetics and Potential Drug-Drug Interactions: A Comparison of
Evidence-Based Literature and Package Insert Information. Am J Geriatr Pharmacother. 2012 Jan 27. [Epub ahead of print] PubMed PMID: 22285509.
6 Biomedical Informatics](https://image.slidesharecdn.com/boyce-cshals-2012-linked-spls-final-120228072423-phpapp02/85/Boyce-cshals-2012-linked-sp-ls-final-6-320.jpg)
![Is there any requirement that the inserts
be kept up to date?
• Yes - 2006 regulations explicitly require that studies of
drug-drug interactions, metabolic pathways, and special
populations be discussed [1]
1. FDA. Code of Federal Regulations 21 Part 201.56, chapter Requirements on content and format of labeling for human prescription drug and biological
products. Washington, DC: US Government Printing Office, 2010. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=201.57
7 Biomedical Informatics](https://image.slidesharecdn.com/boyce-cshals-2012-linked-spls-final-120228072423-phpapp02/85/Boyce-cshals-2012-linked-sp-ls-final-7-320.jpg)
![Why are package inserts important?
• Package inserts are primarily intended to be a reference
for prescribing clinicians [1]
1. Marroum PJ, Gobburu J. The product label: how pharmacokinetics and
pharmacodynamics reach the prescriber. Clin Pharmacokinet. 2002;41(3):161-169.
2. Ko, Y. et al. Prescribers’ knowledge of and sources of information for potential drug-
drug interactions: a postal survey of US prescribers. Drug Saf. 31, 525-536 (2008).
8 Biomedical Informatics](https://image.slidesharecdn.com/boyce-cshals-2012-linked-spls-final-120228072423-phpapp02/85/Boyce-cshals-2012-linked-sp-ls-final-8-320.jpg)







![The Structured Product Label (SPL)
standard makes this possible
• All package inserts for currently marketed drugs
are available in this format [1-3]
1. http://www.fda.gov/OHRMS/DOCKETS/98fr/FDA-2005-N-0464-gdl.pdf
2. http://www.fda.gov/ForIndustry/DataStandards/StructuredProductLabeling/default.htm
3. http://dailymed.nlm.nih.gov/dailymed/downloadLabels.cfm
17 Biomedical Informatics](https://image.slidesharecdn.com/boyce-cshals-2012-linked-spls-final-120228072423-phpapp02/85/Boyce-cshals-2012-linked-sp-ls-final-17-320.jpg)