The document discusses body fluid compartments and their properties. It notes that:
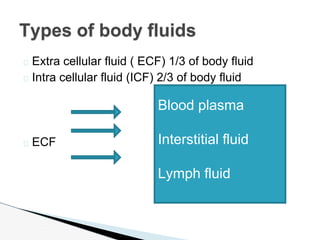
- The body is composed of water, minerals, proteins, and fats. Fluids are divided into extracellular fluid (ECF) and intracellular fluid (ICF). ECF includes blood plasma, interstitial fluid, and lymph.
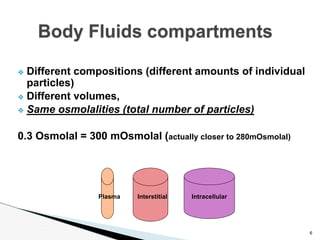
- Fluid compartments have different sizes, volumes, and compositions, but the same number of particles (300 million) per liter. Manipulating one compartment affects others.
- Compositions vary between compartments but osmolalities remain the same. Water moves between compartments via osmosis or hydrostatic pressure. Cell membranes are selectively permeable and maintain concentration gradients.



















![[Ca++]i⇑
Voltage-gated channel](https://image.slidesharecdn.com/bodyfluid-230627115600-fc67836e/85/bodyfluid-pptx-22-320.jpg)



