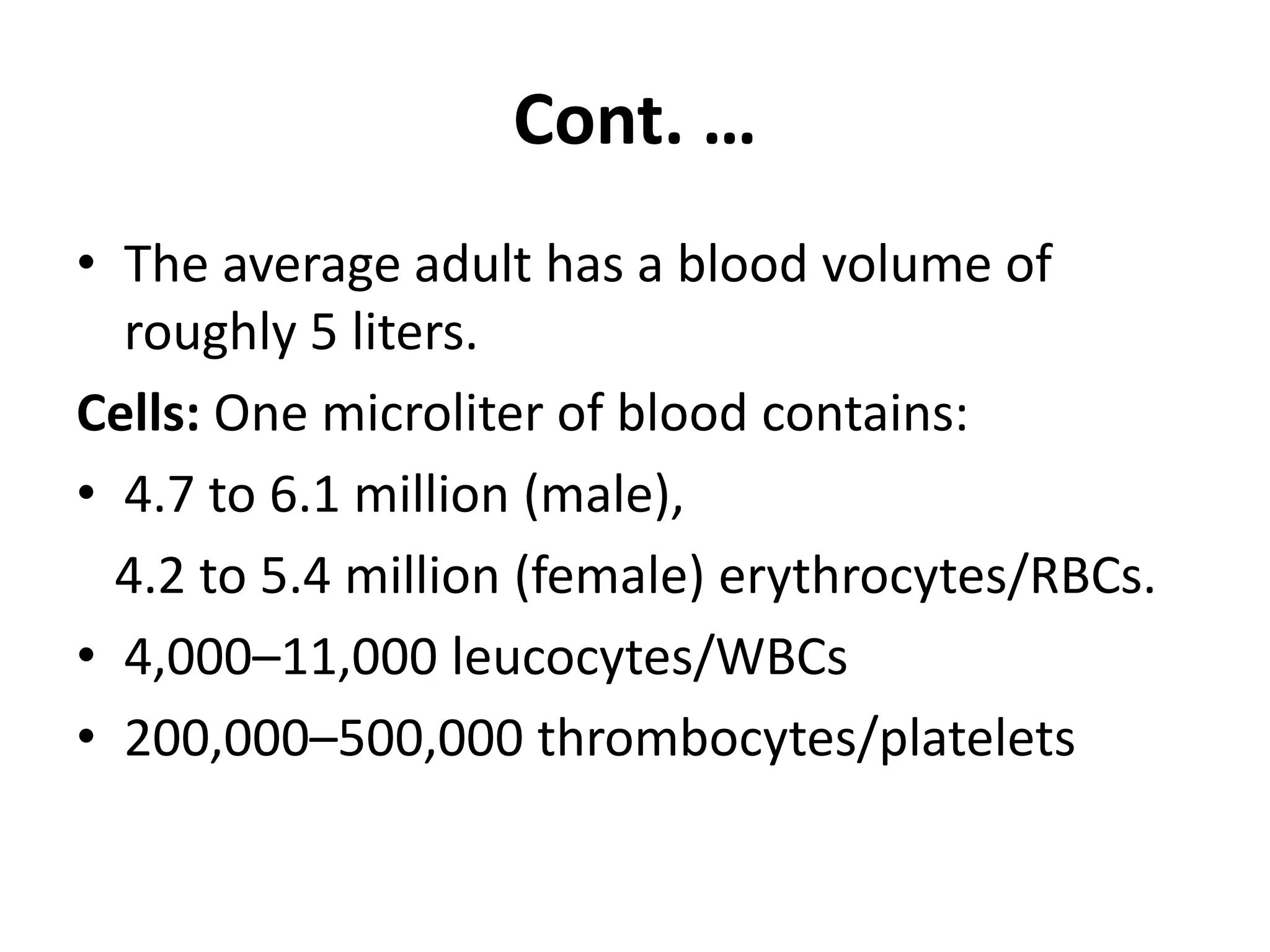
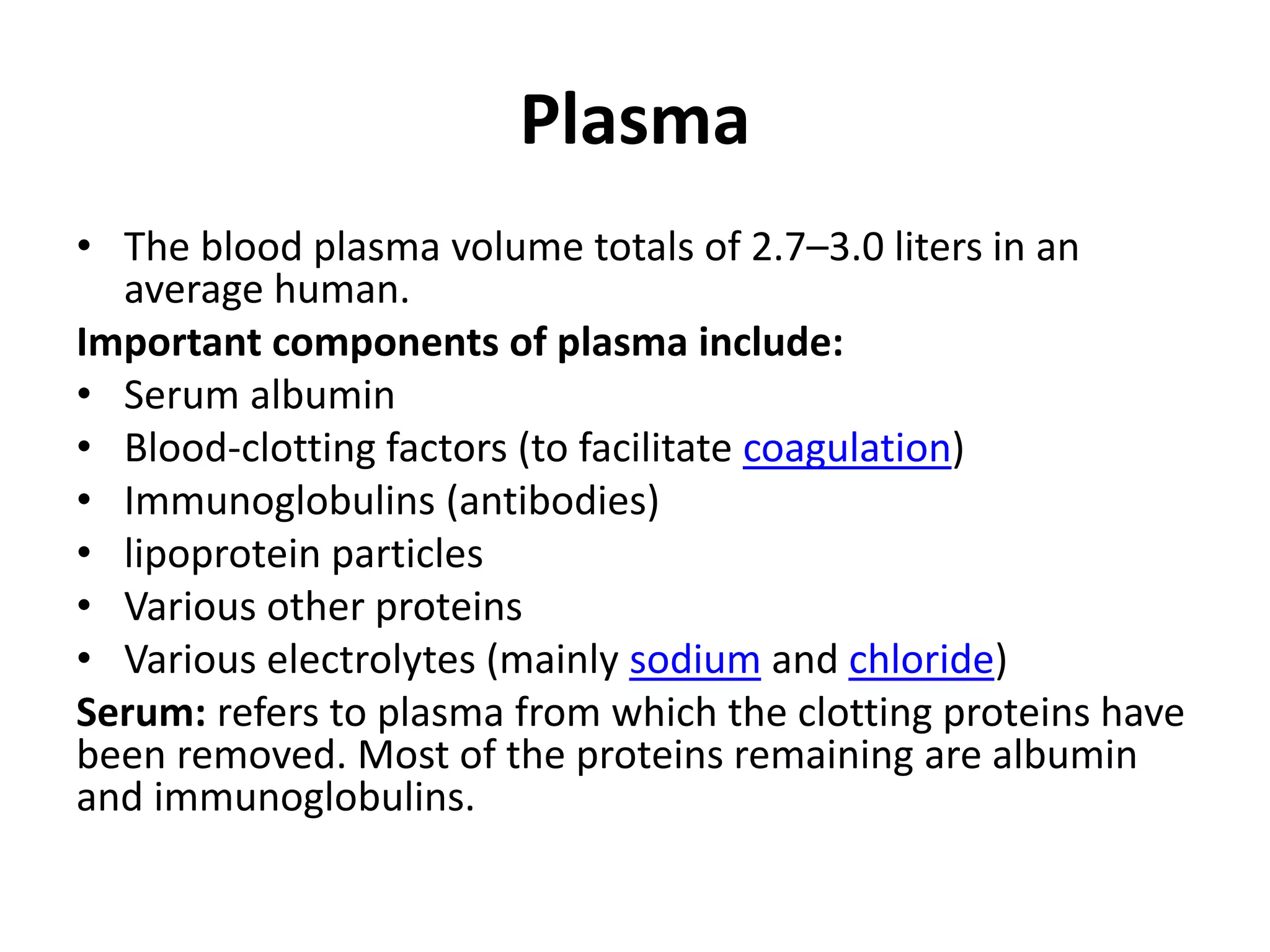
This document provides instructions for examining blood samples. It describes the components of blood and how to collect blood through either capillary or venous methods. Detailed procedures are provided for making wet blood preparations, thick blood films, and thin blood films in order to examine blood cells and detect parasites. Quality controls and common problems in blood film preparation are also discussed.