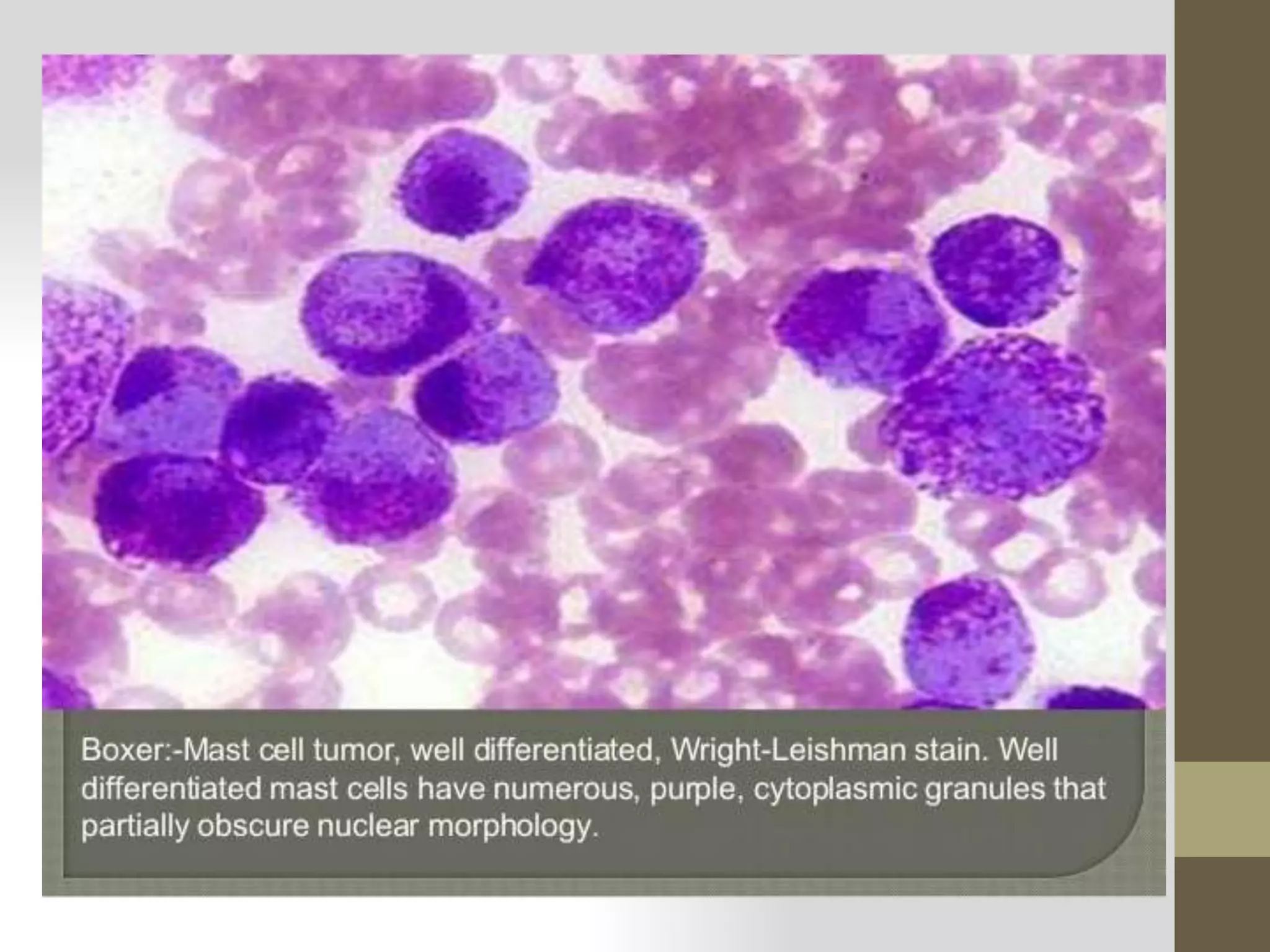
This document provides information on preparing blood smears with different staining methods. It discusses preparing thin and thick blood smears using various techniques like the slide method, cover glass method, and spin method for thin smears. For thick smears, a large drop of blood is spread on the slide. Various staining methods are described like Leishman stain, Giemsa stain, Wright stain, and Field stain. Proper preparation, fixation in methanol or ethanol, and staining are required to visualize blood cells and parasites under the microscope. Reticulocyte staining is also briefly mentioned.