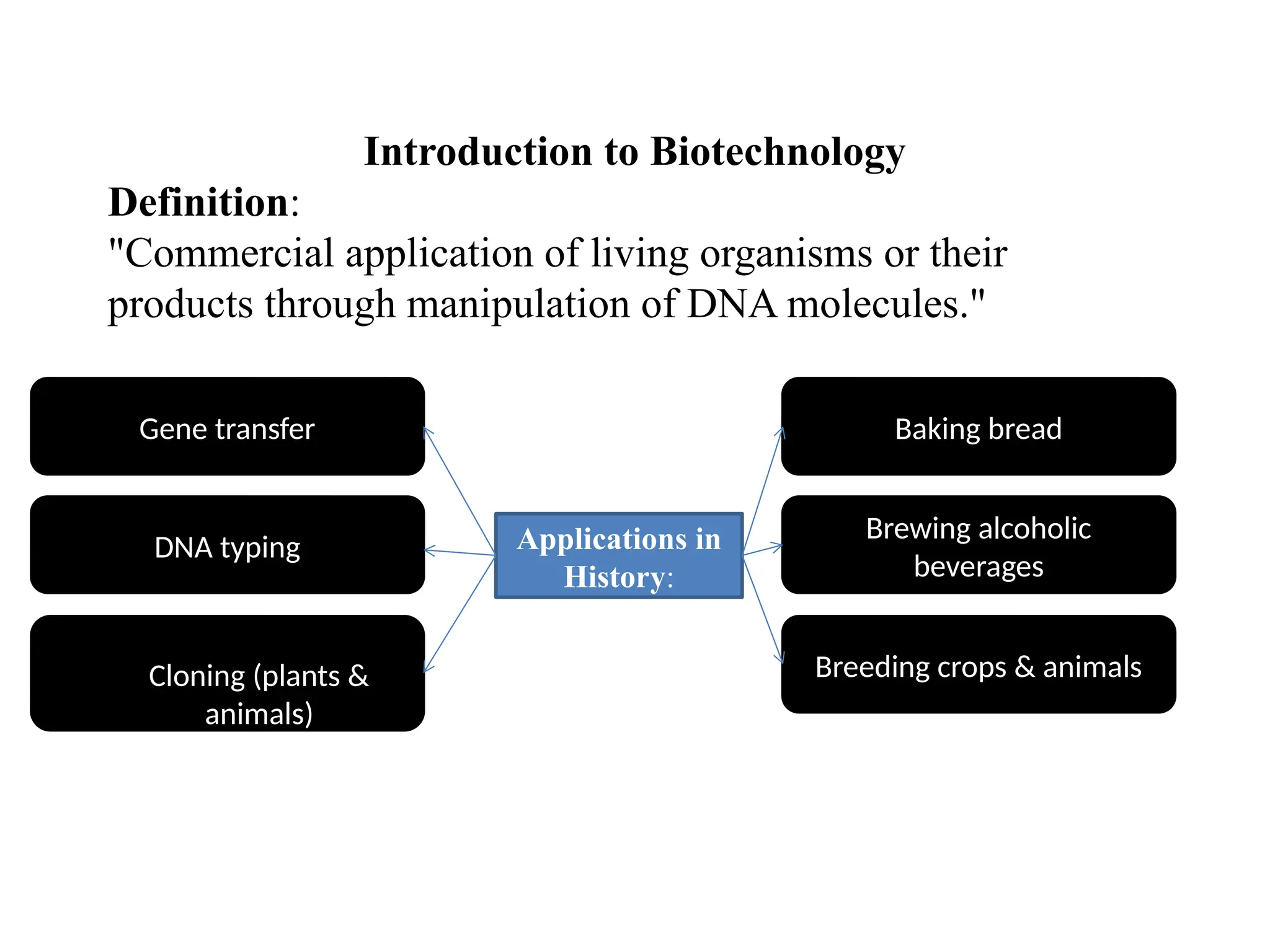
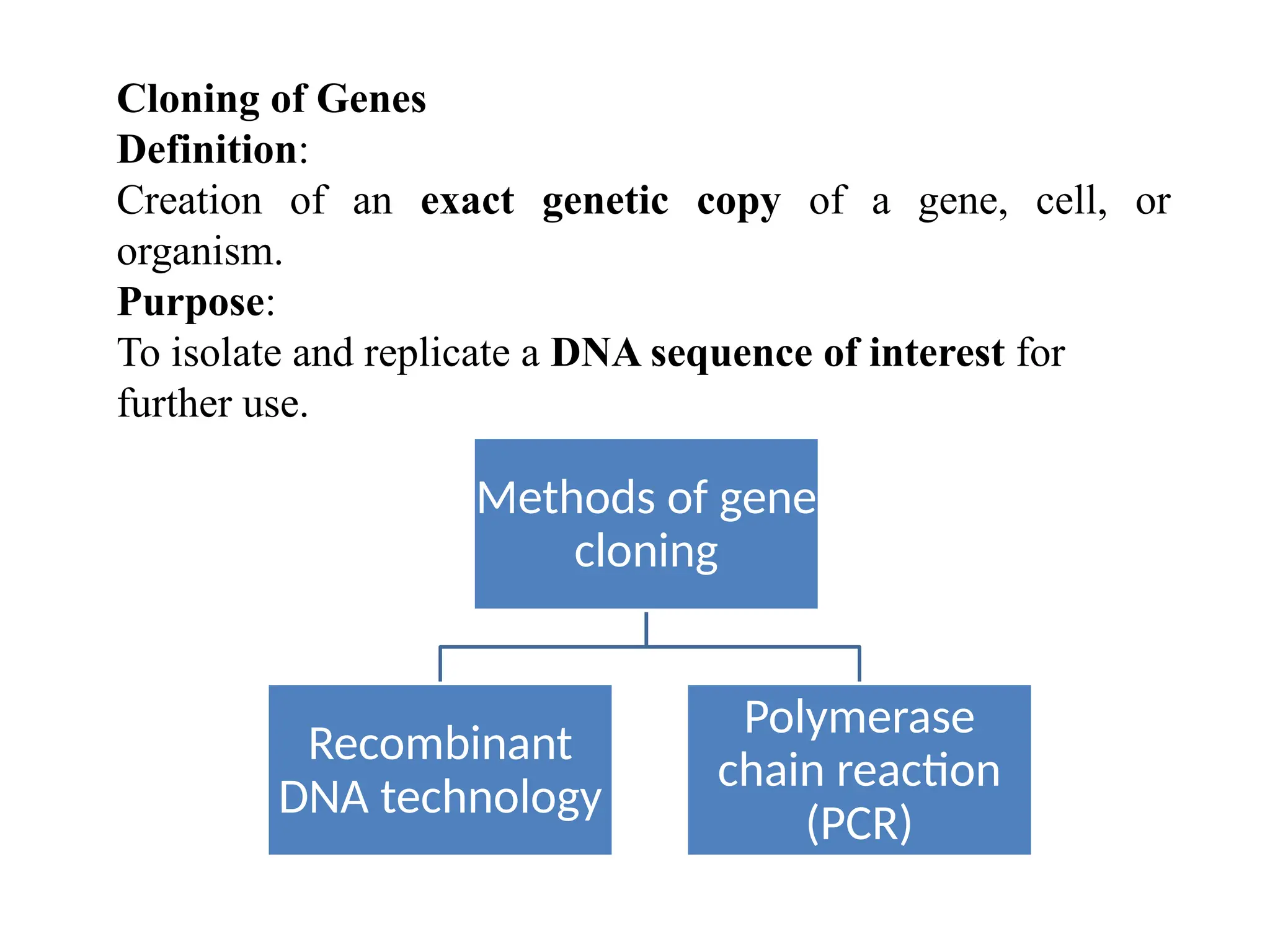
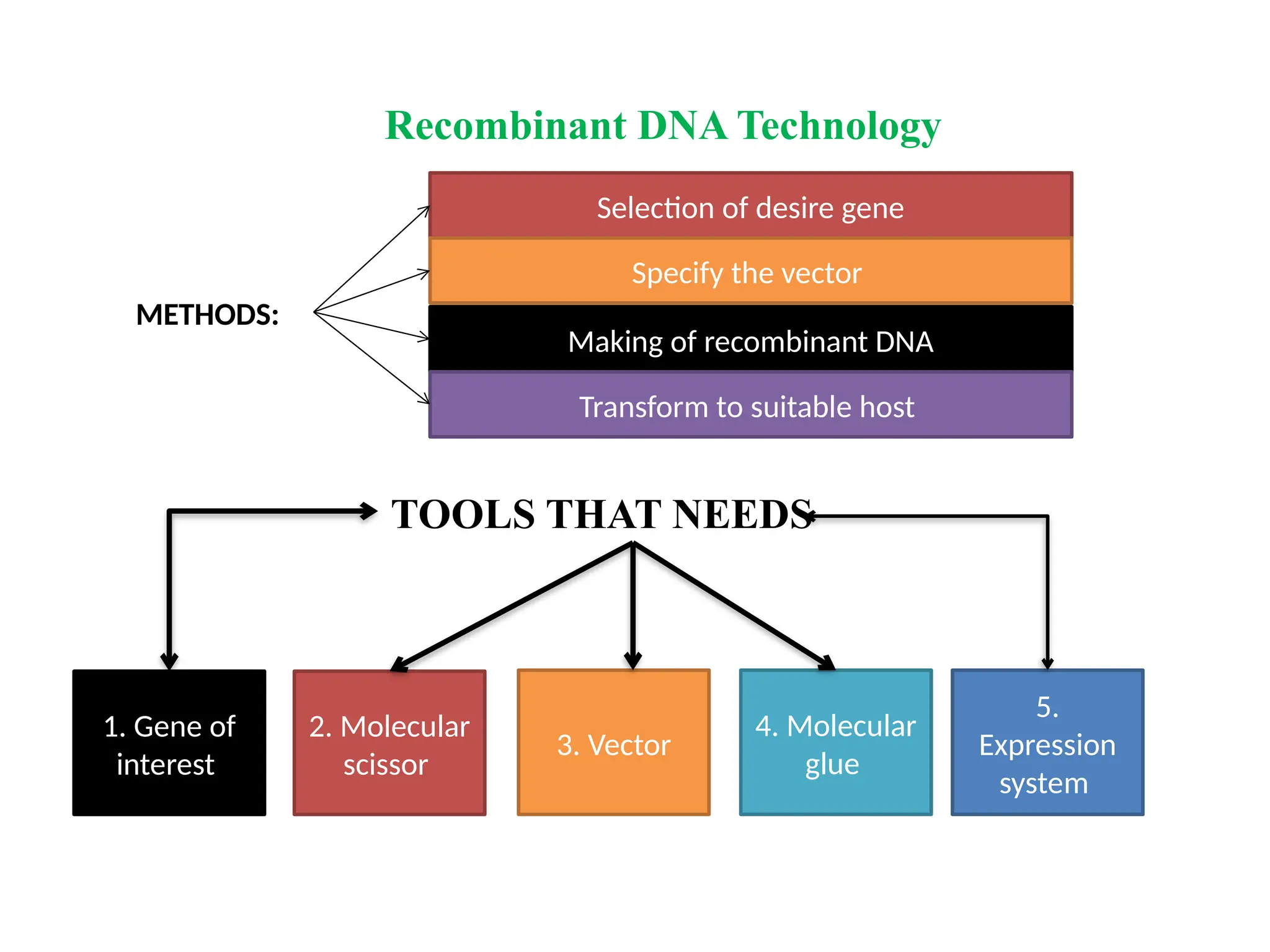
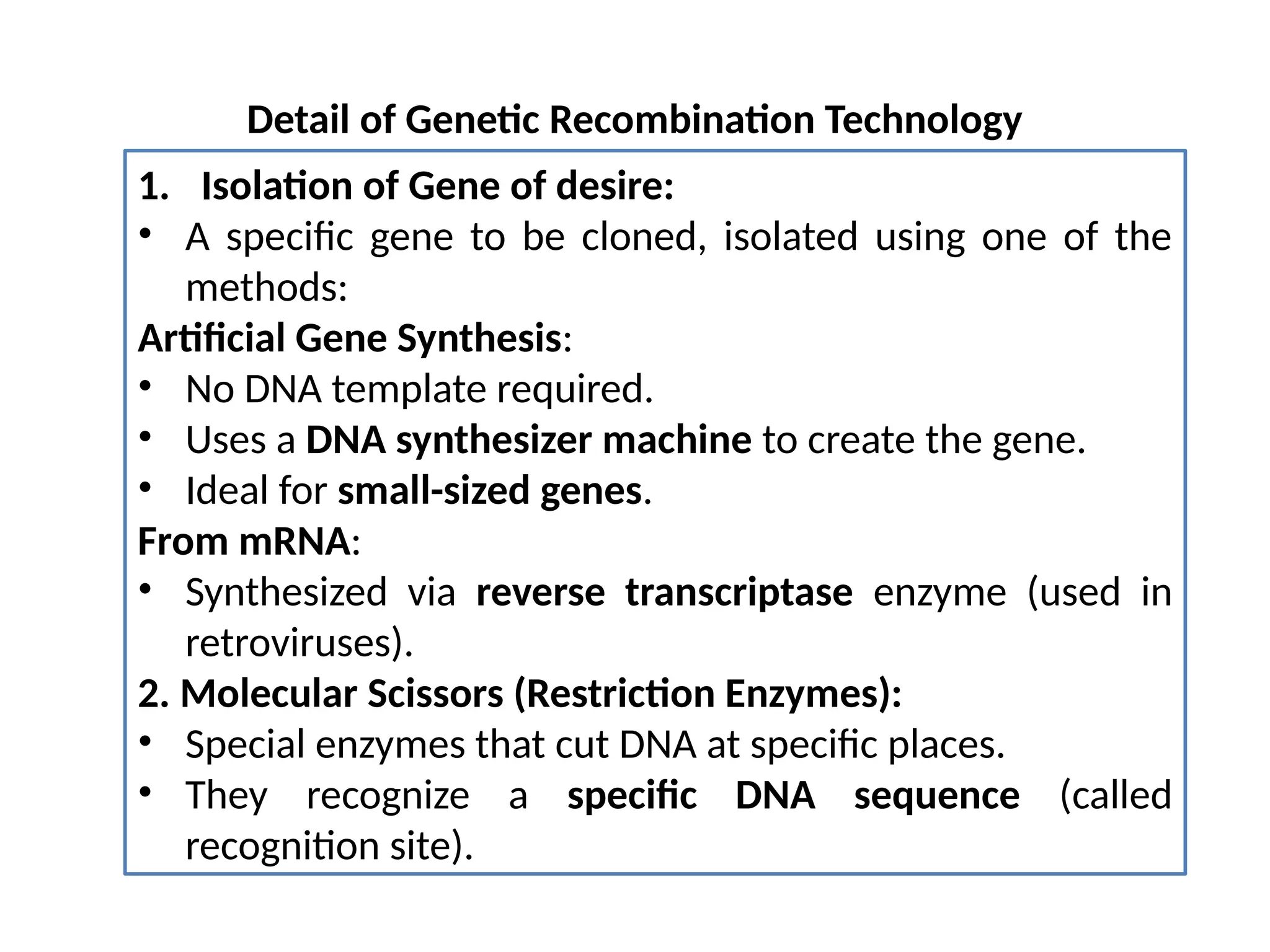
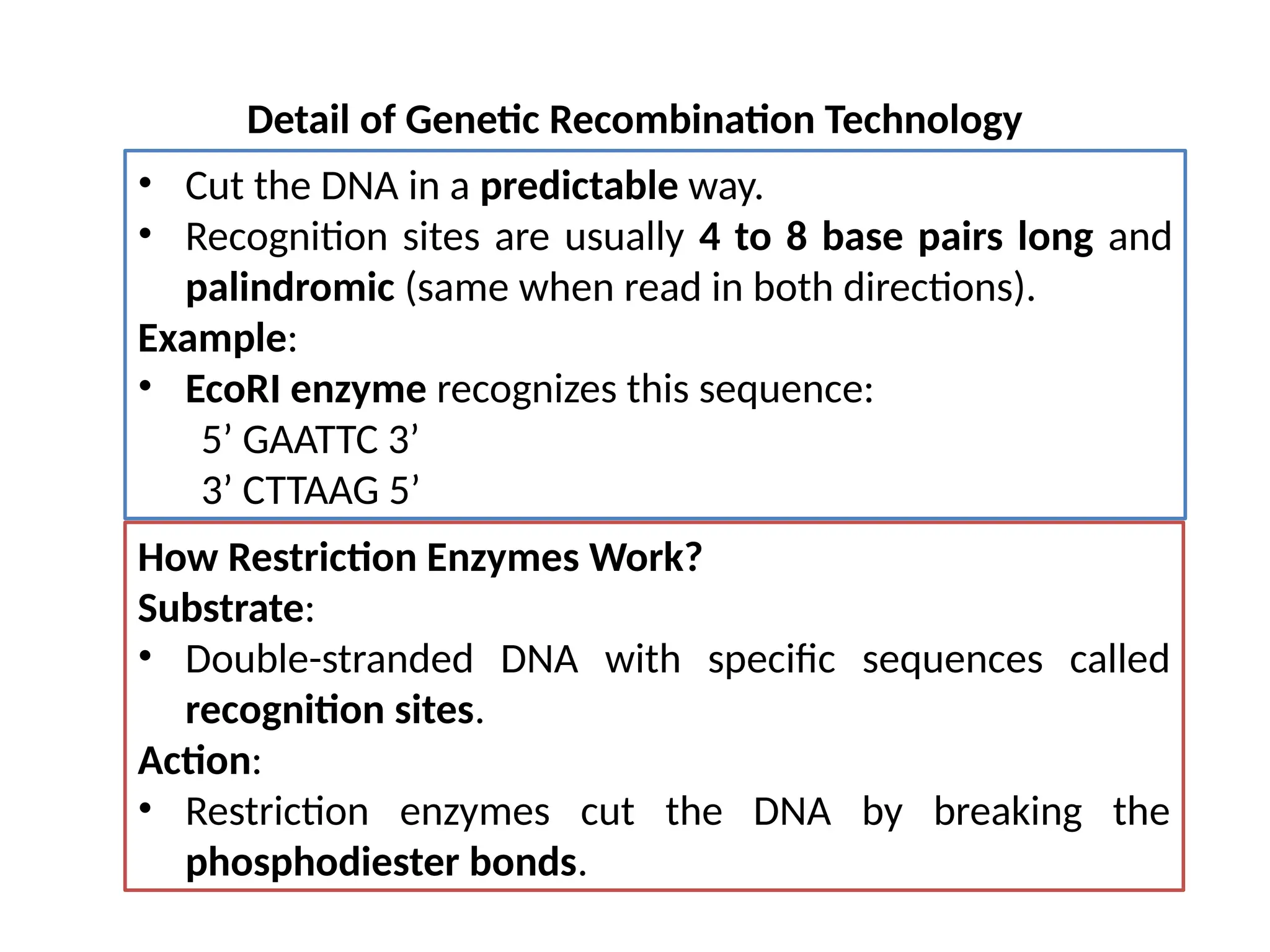
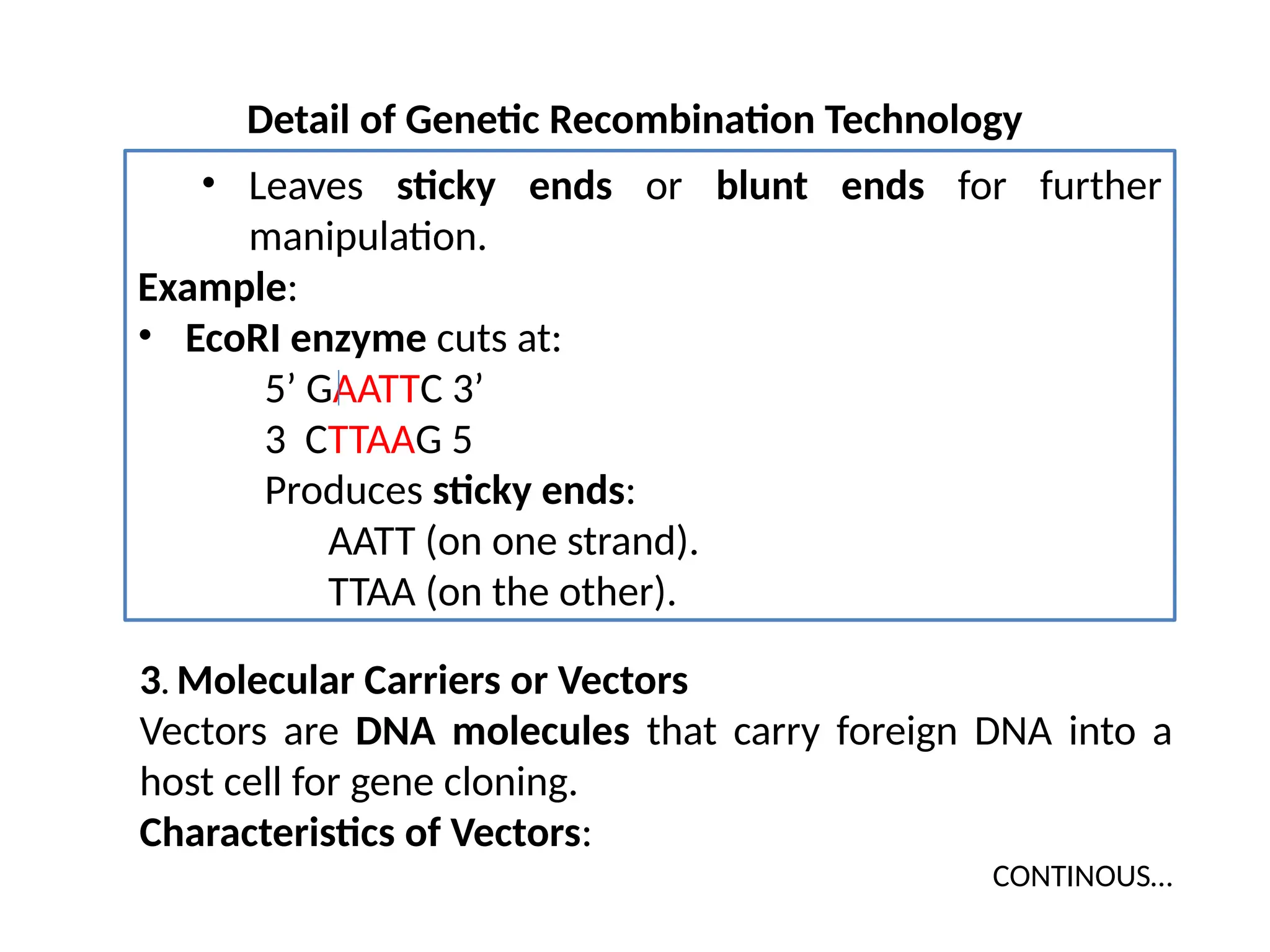
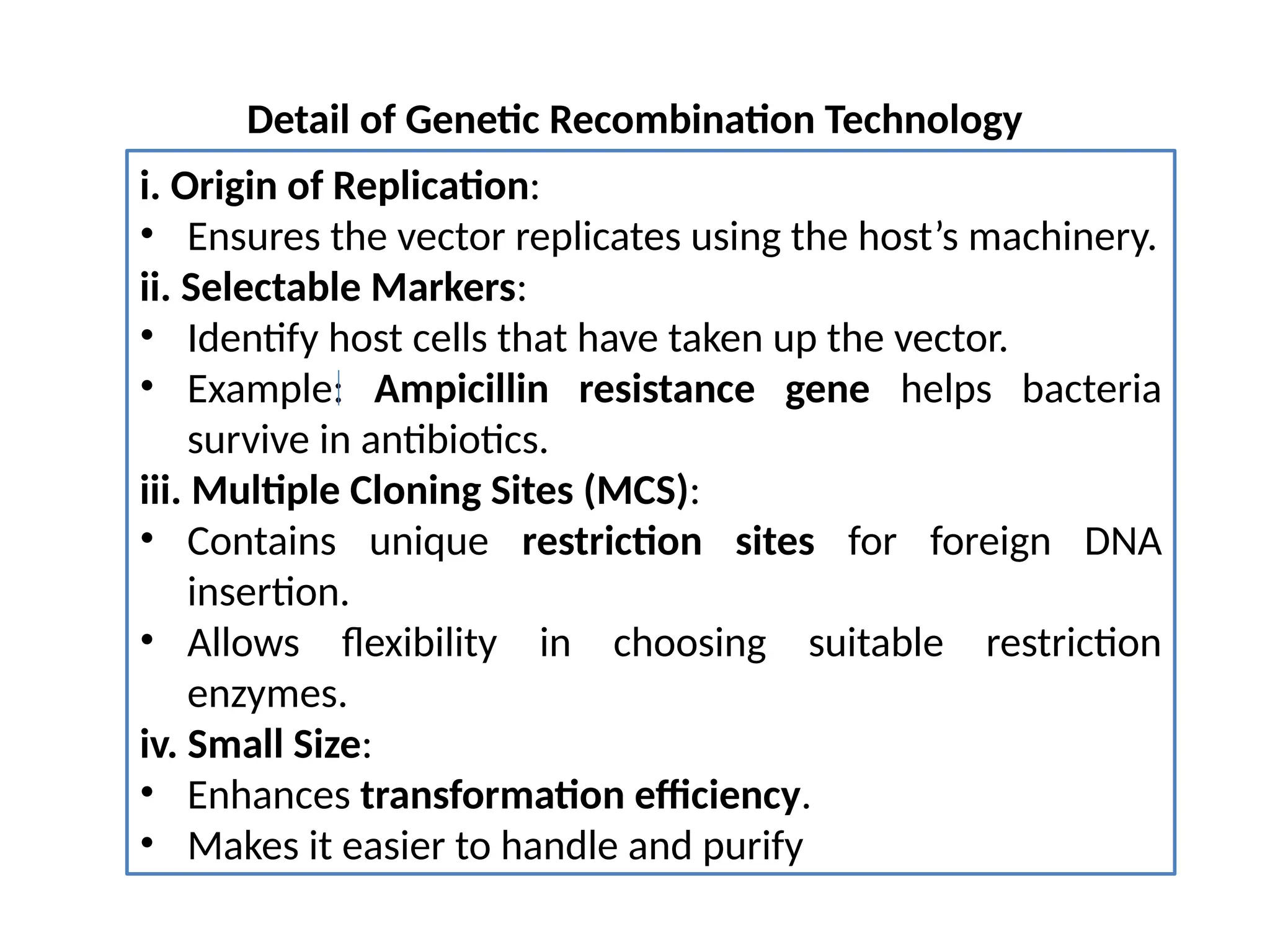
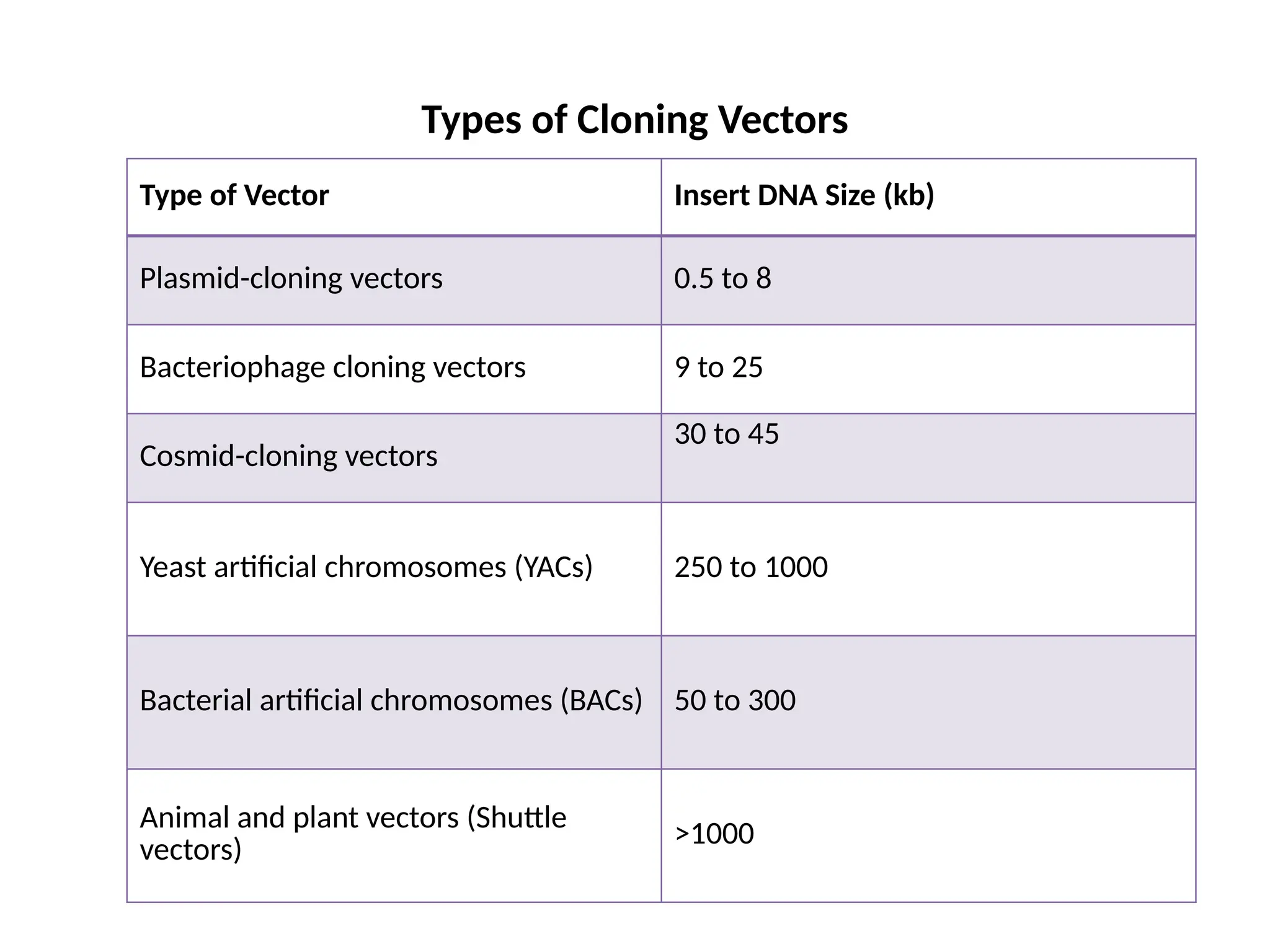
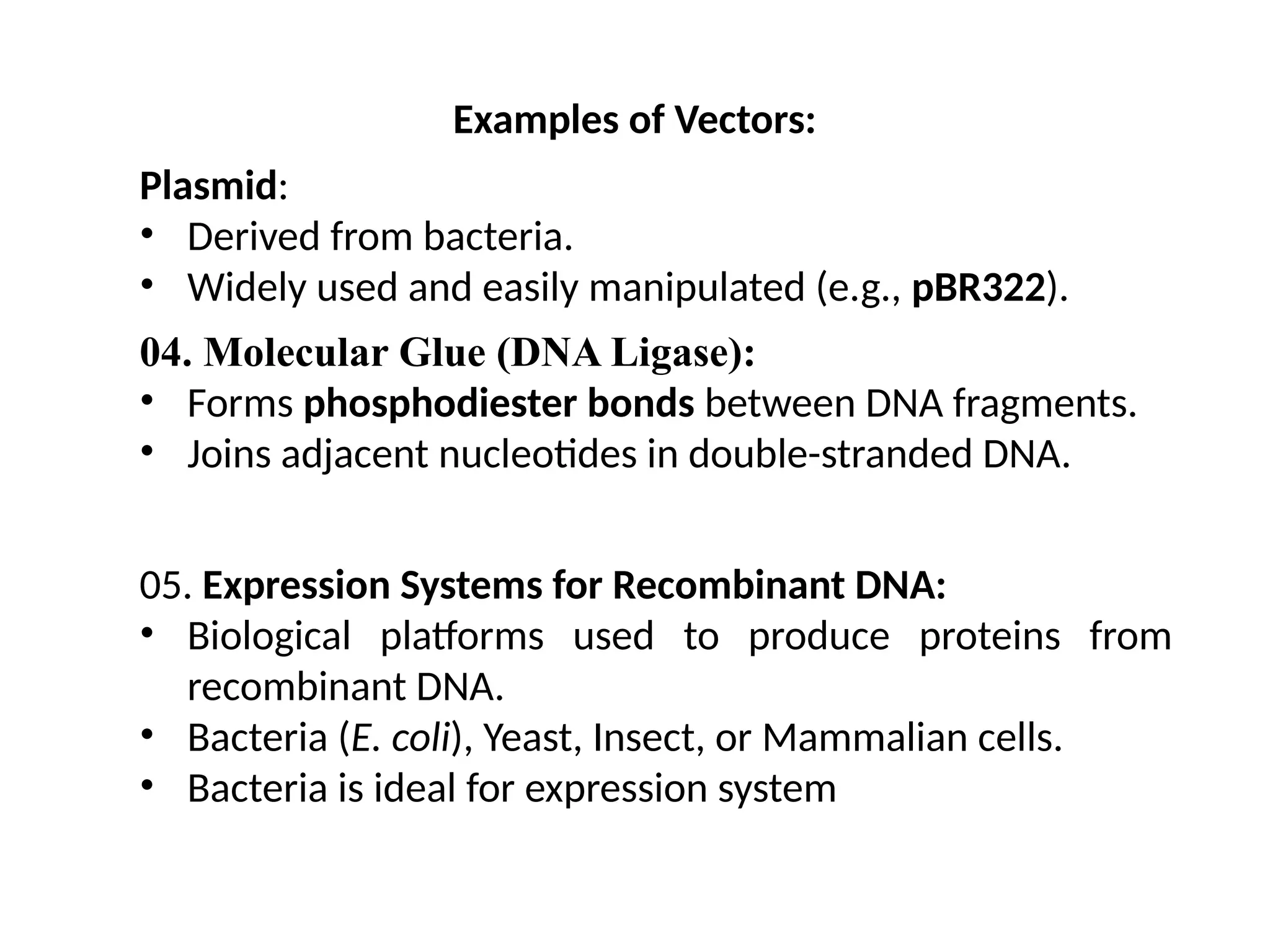
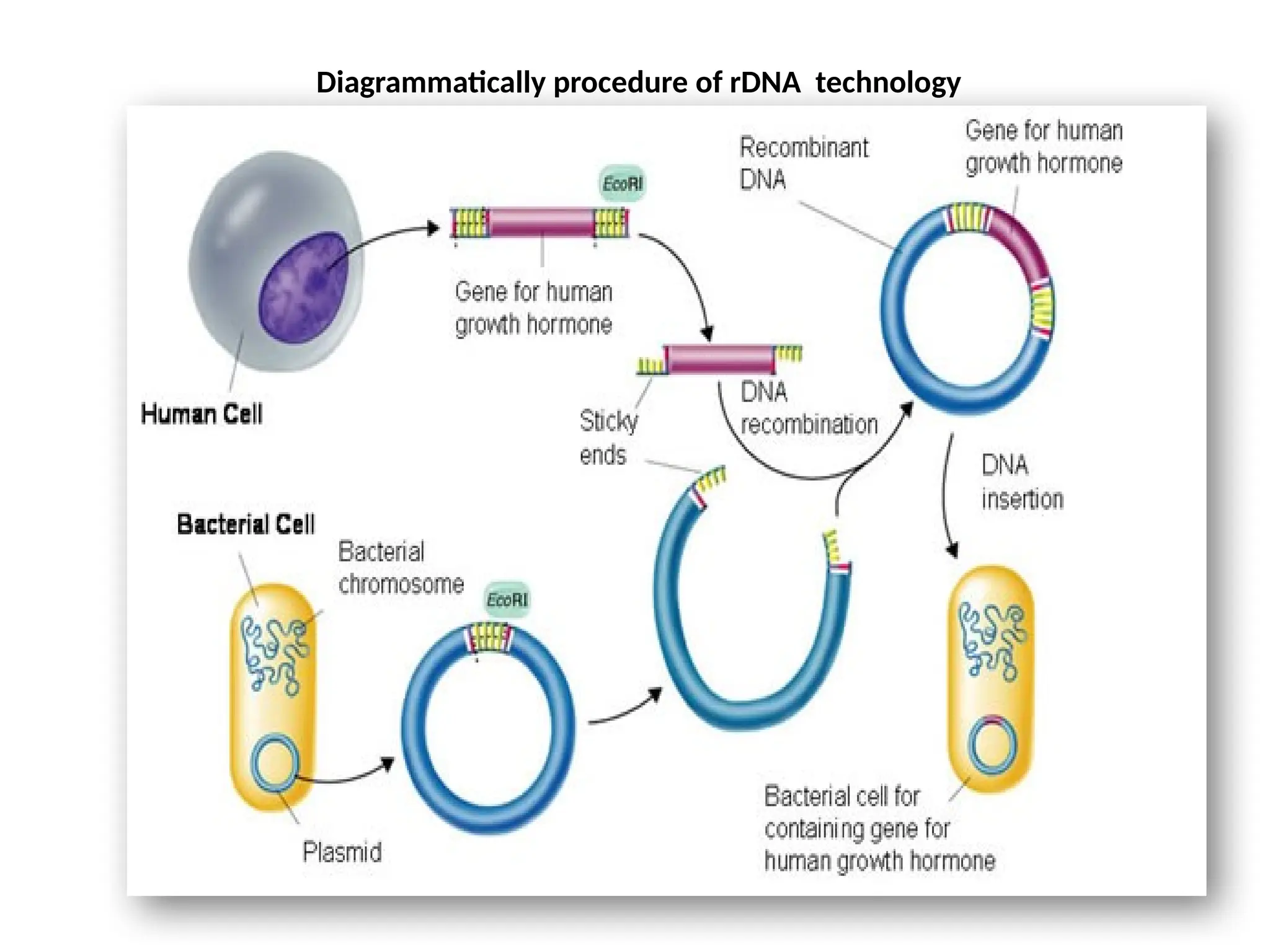
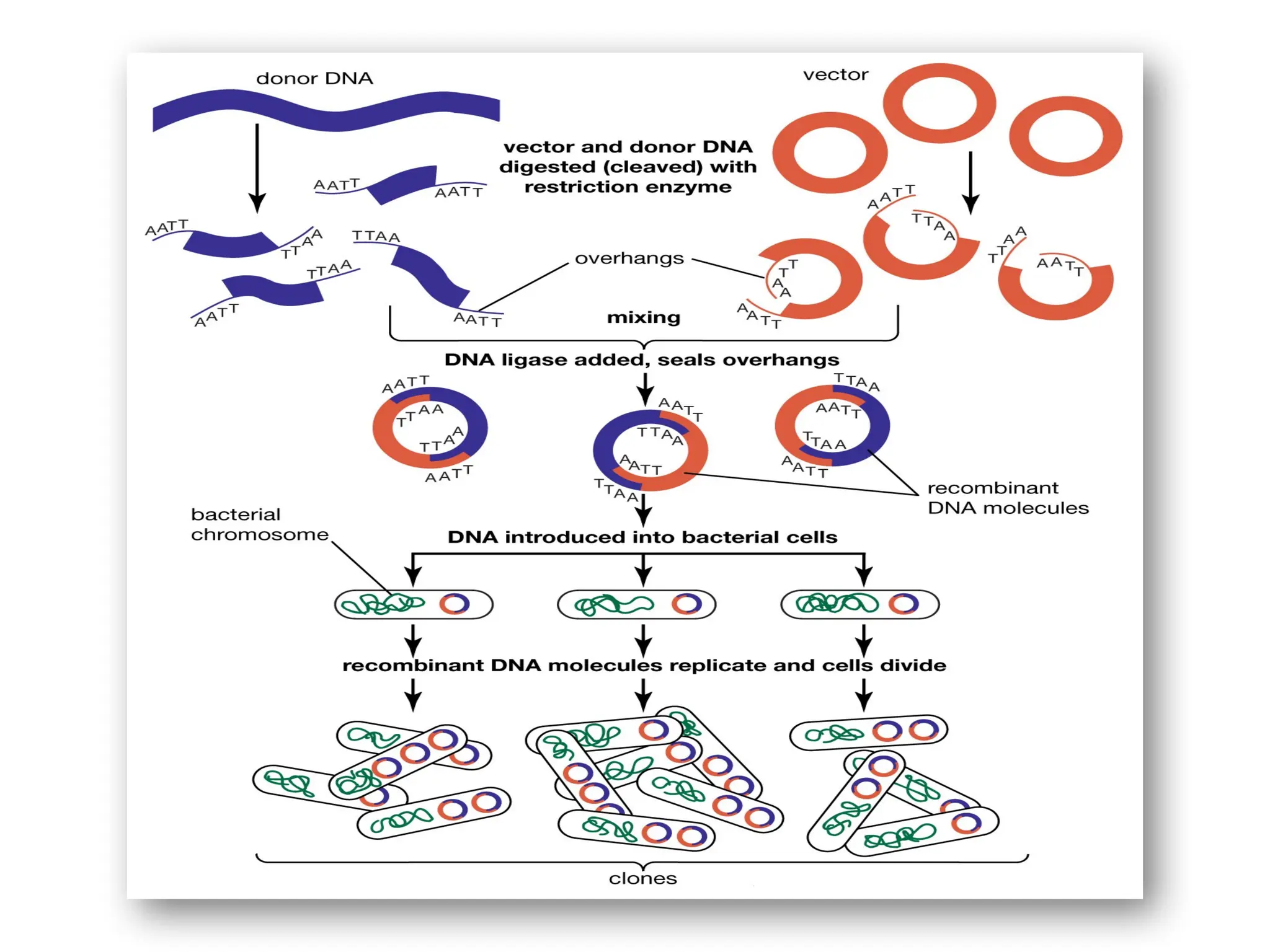
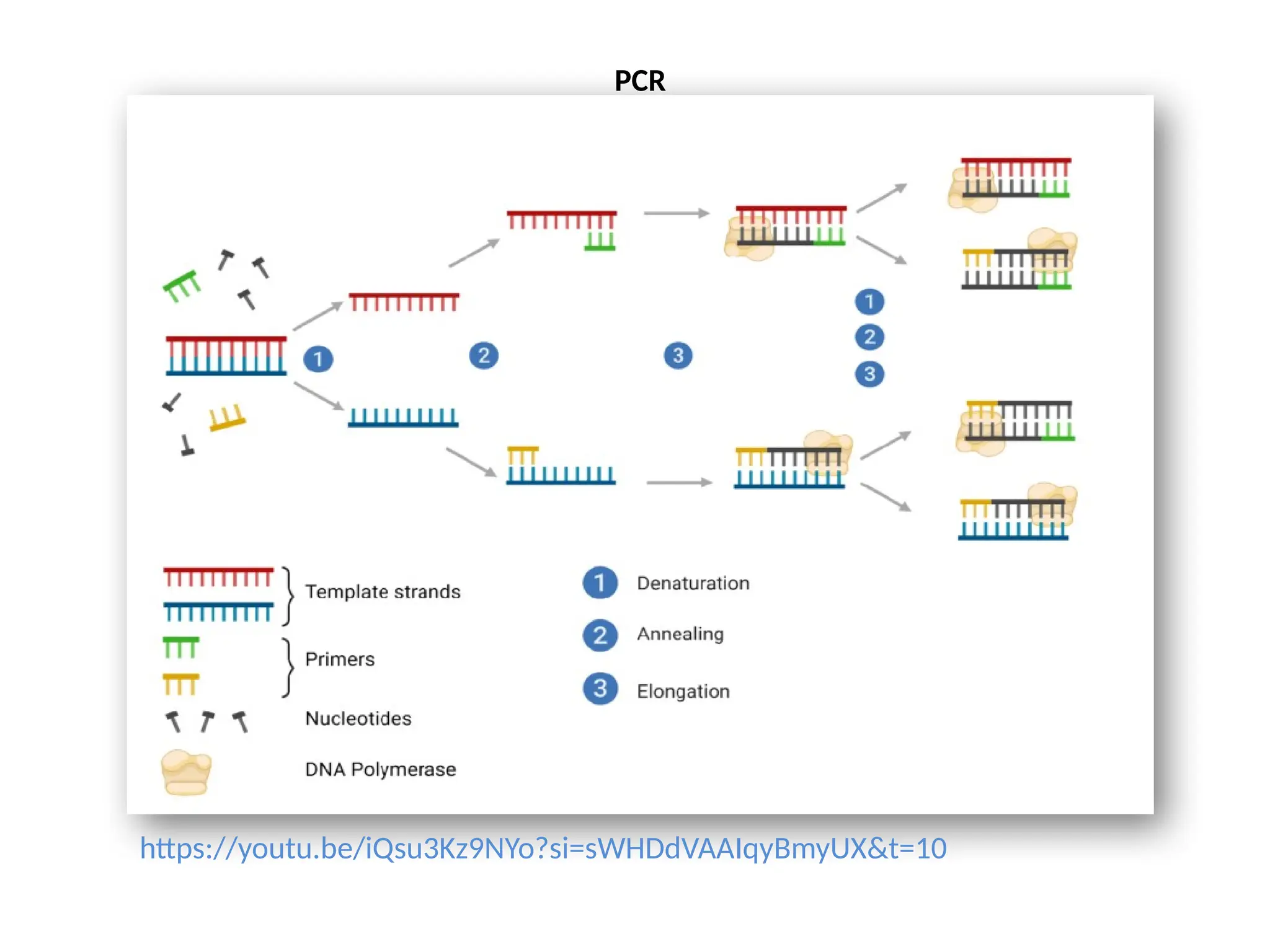
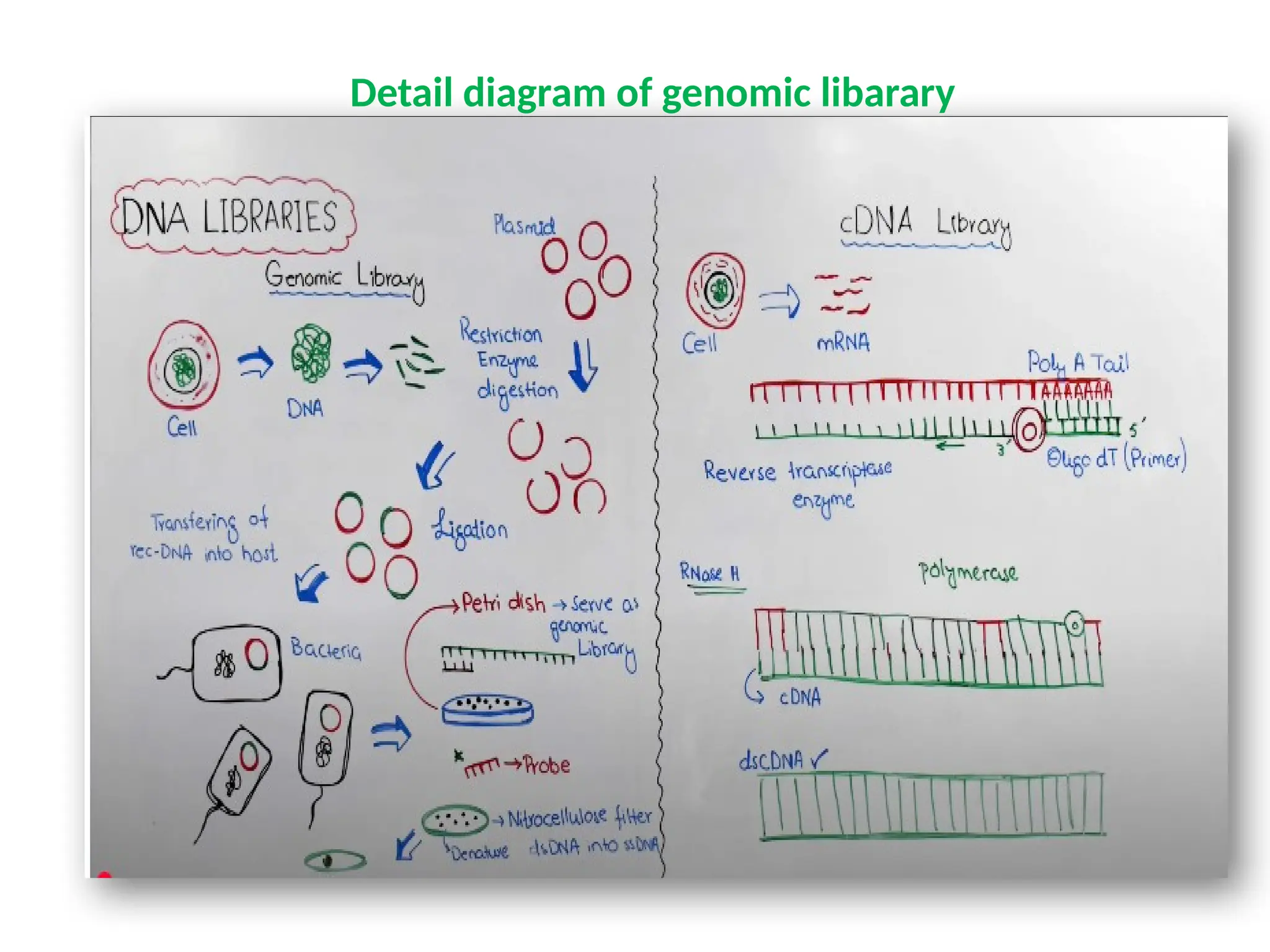
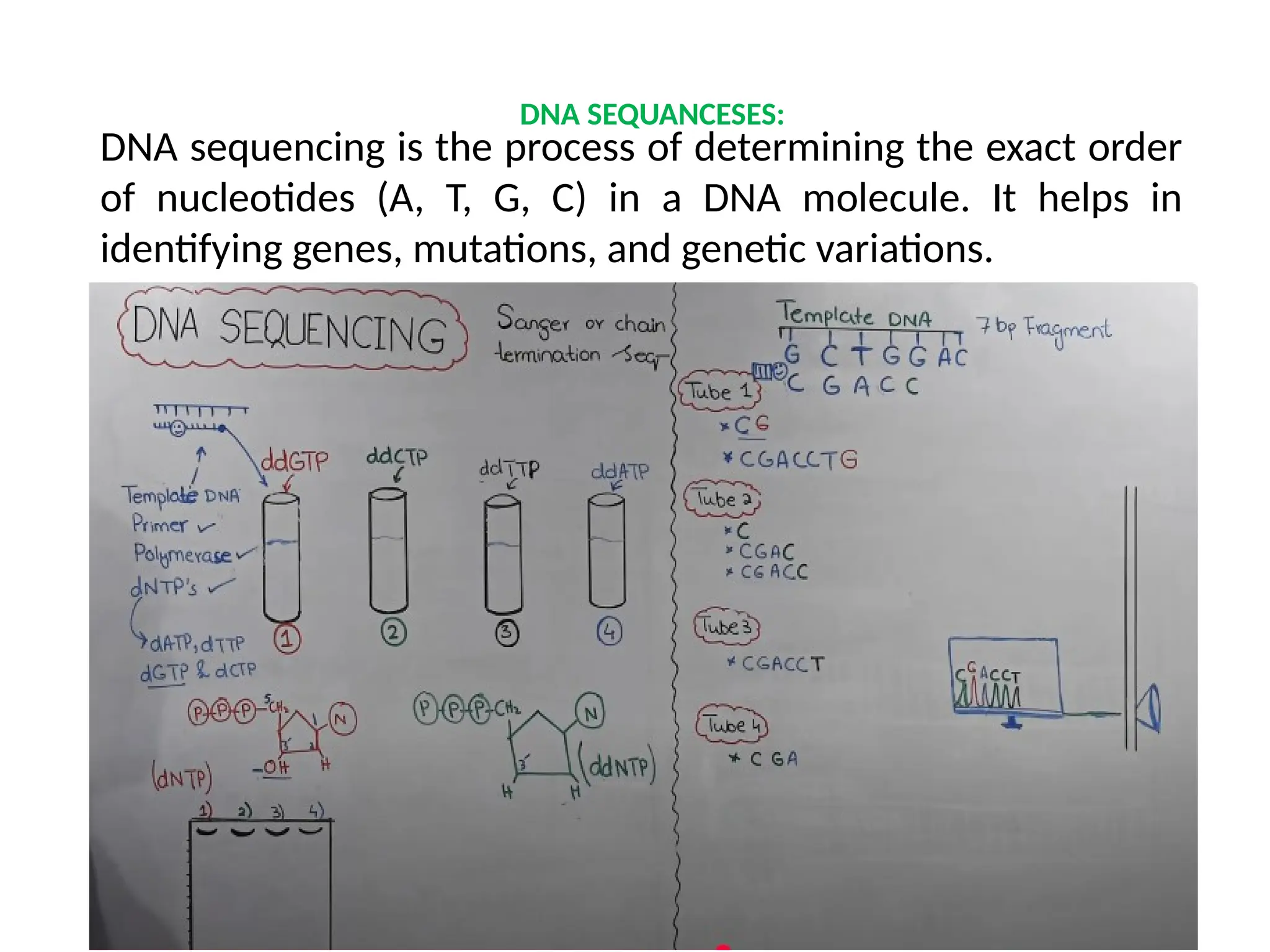
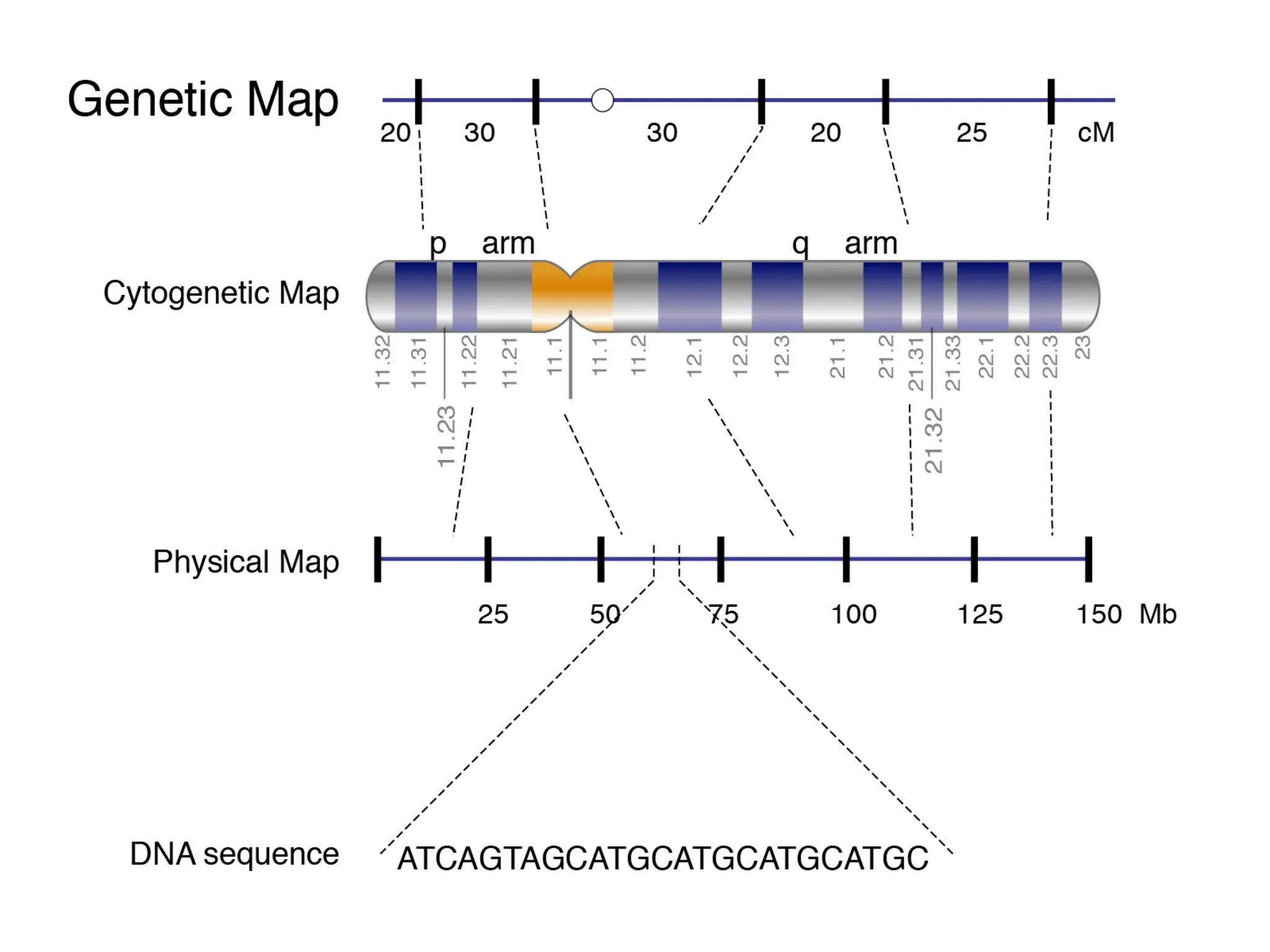
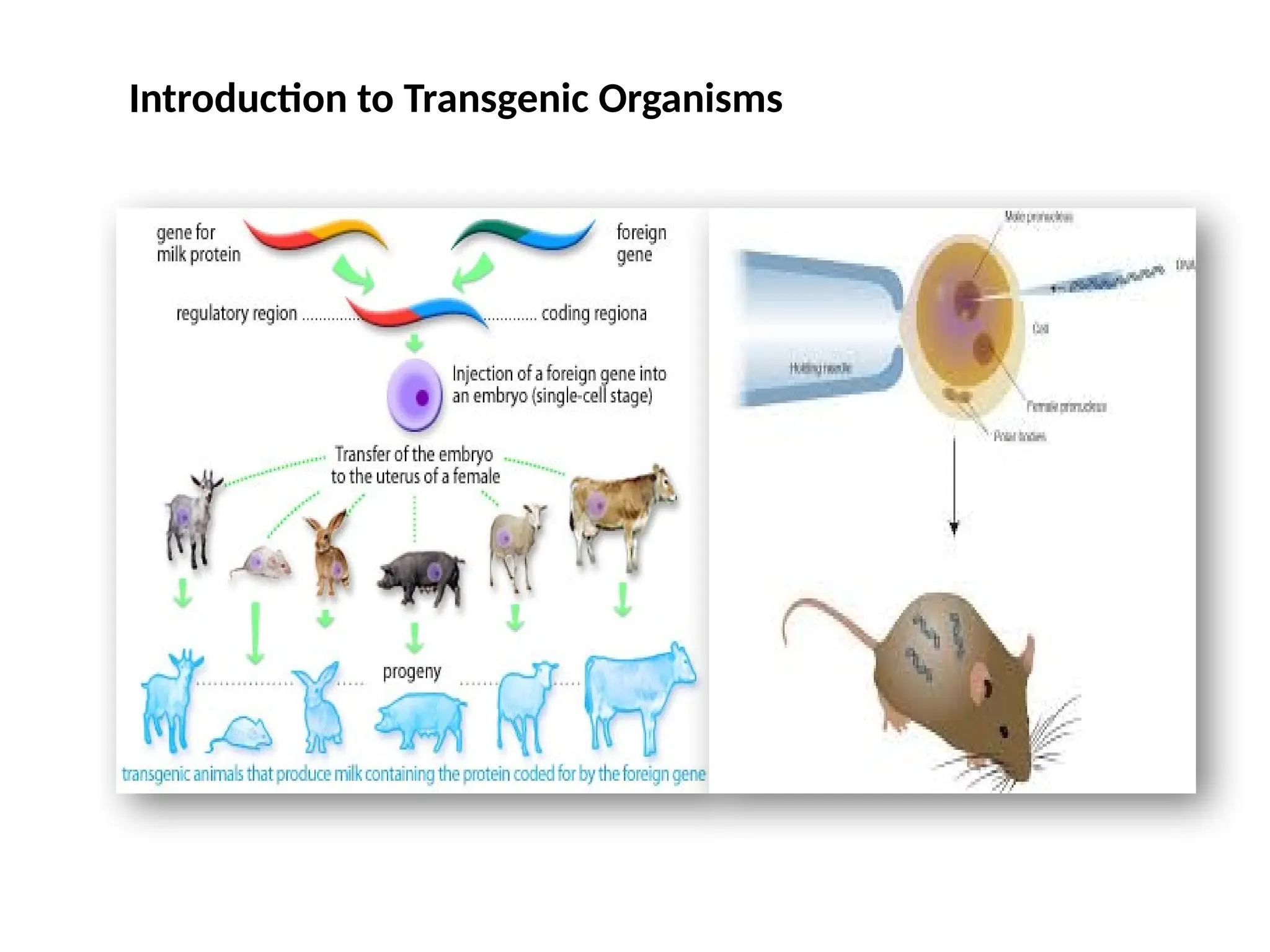
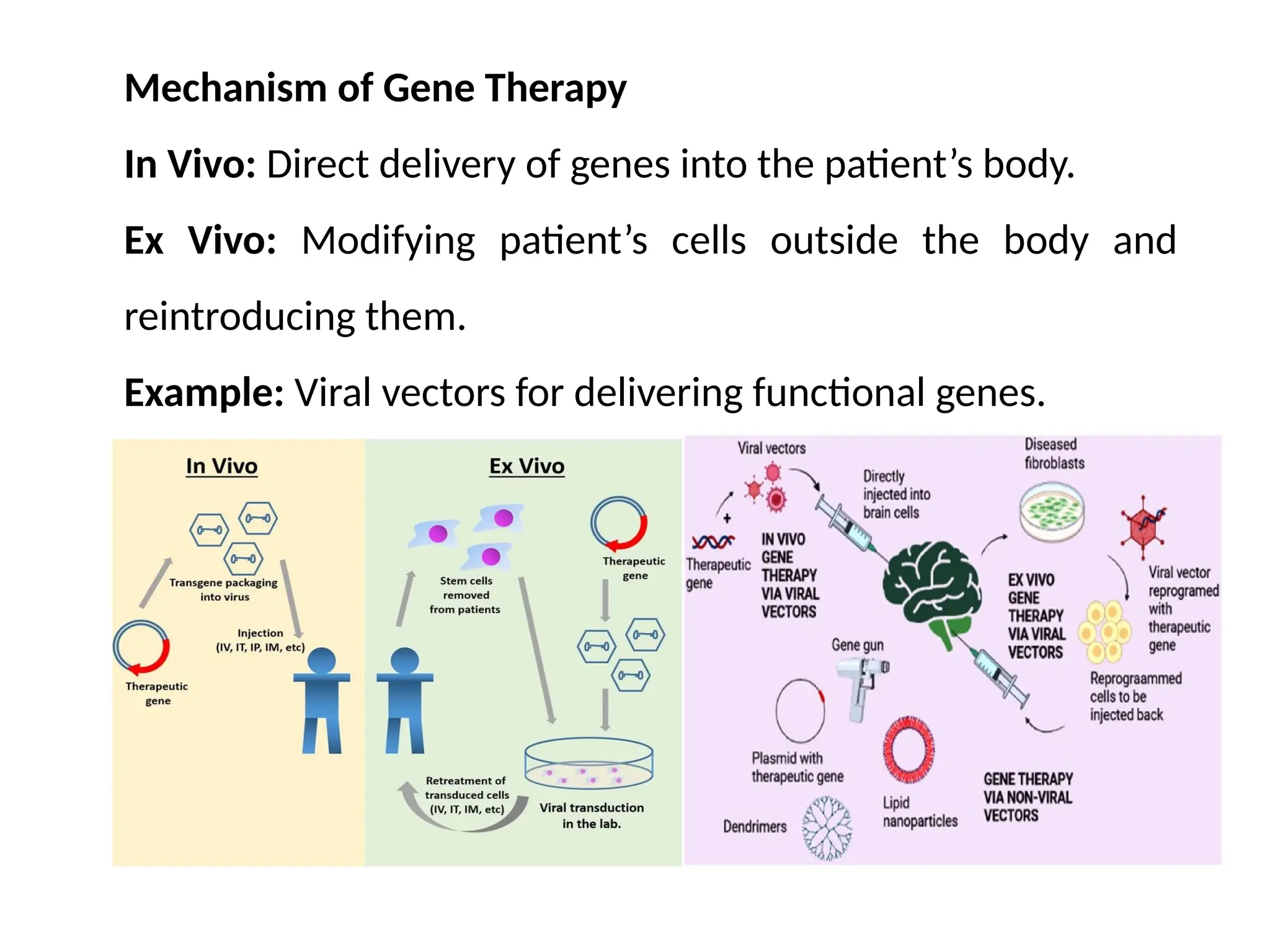
The document provides an extensive overview of biotechnology, covering key concepts such as gene cloning, genomics, DNA sequencing, and the creation of transgenic organisms. It details methods for genetic manipulation, including PCR, the construction of genomic libraries, and RFLP DNA fingerprinting, while outlining the applications of these techniques in medicine, agriculture, and genetic research. Additionally, it highlights the importance and impact of the Human Genome Project and tissue culture in biotechnology.