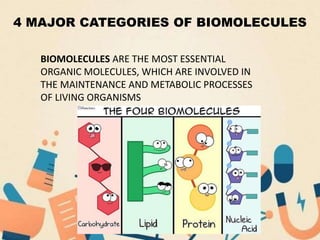
The document discusses the four main categories of biomolecules: carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates include monosaccharides (glucose, fructose, galactose), disaccharides (lactose, maltose, sucrose) and polysaccharides. Lipids are insoluble and include fats, oils, phospholipids, steroids and waxes. Proteins are made of amino acids and can function as enzymes or hormones. Nucleic acids DNA and RNA carry genetic information and instructions for cell functioning.