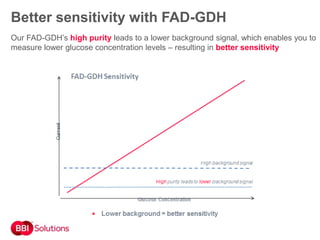
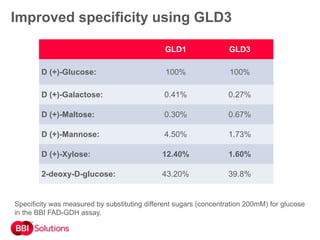
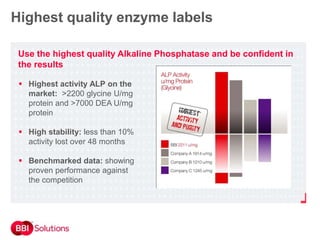
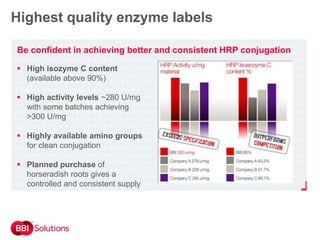
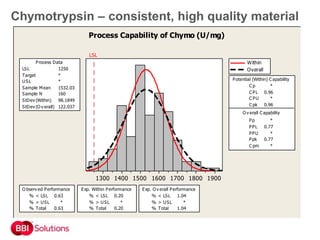
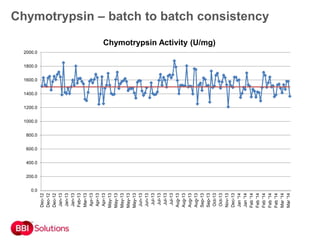
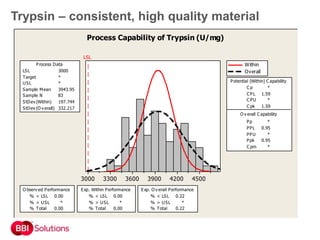
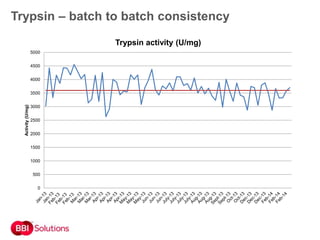
BBI is a leading supplier of enzymes for diagnostic and research applications. They have over 50 years of experience producing high-quality enzymes. Some of their key products mentioned in the document include glucose oxidase, glucose dehydrogenase, alkaline phosphatase, and horseradish peroxidase. The document provides details on the performance, quality control, and benefits of these enzymes. It emphasizes BBI's long experience producing enzymes like chymotrypsin and trypsin, with consistent high quality and batch-to-batch reproducibility.