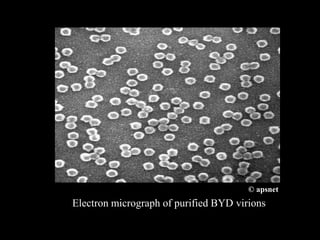
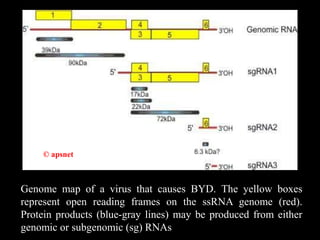
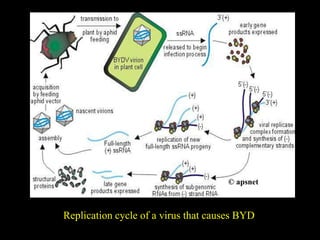
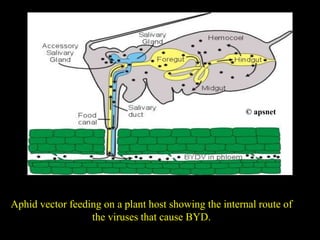
Barley Yellow Dwarf Disease (BYD) is caused by viruses transmitted by aphids, affecting crops like barley, oats, and wheat, leading to symptoms such as stunting and yellowing of leaves. The disease has significant economic implications, with potential yield losses up to 50% from early infections. Management strategies include planting resistant cultivars, monitoring aphid populations, and using seed treatments to control aphid vectors.