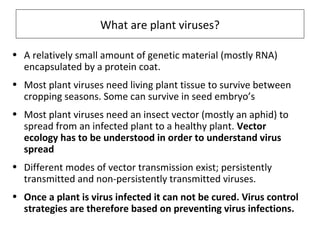
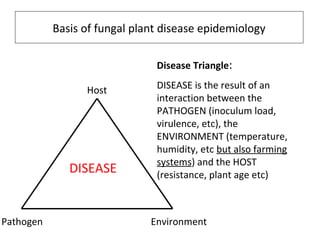
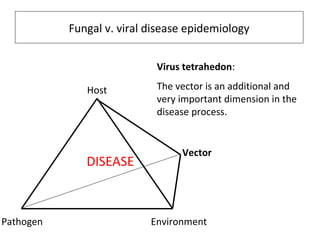
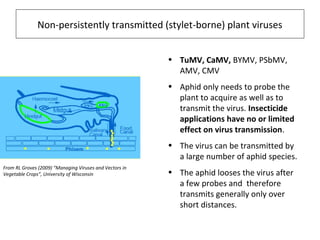
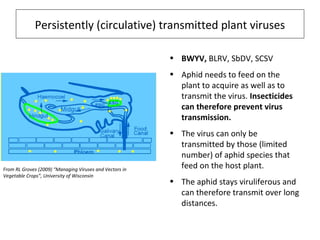
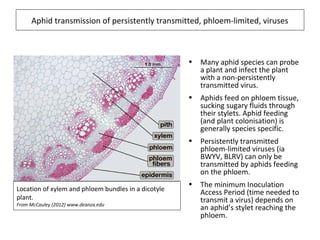
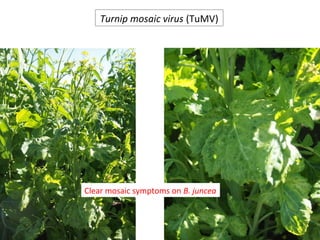
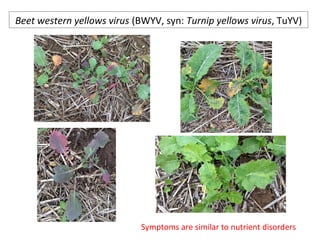
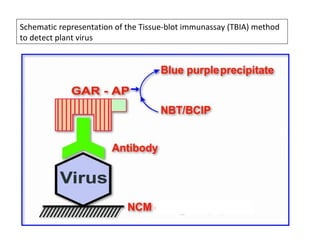
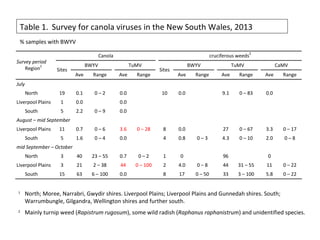
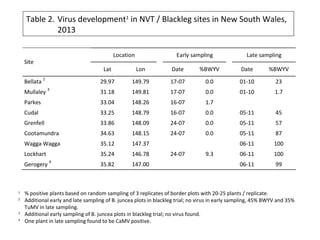
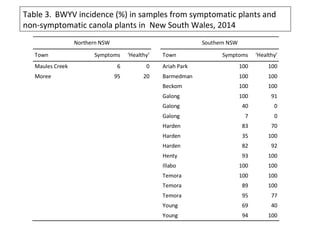
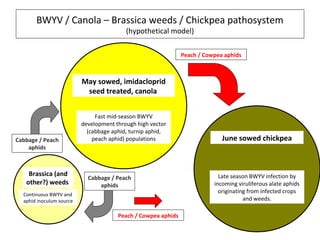
The document discusses the ecology, detection, and occurrence of canola viruses, focusing on their reliance on insect vectors for transmission, particularly aphids. It details different types of plant viruses, their modes of transmission, and control strategies, emphasizing the prevention of virus infections due to the lack of curative measures. Additionally, it highlights the need for reliable diagnostic techniques to distinguish viral infections from other stress factors in canola crops.