Embed presentation

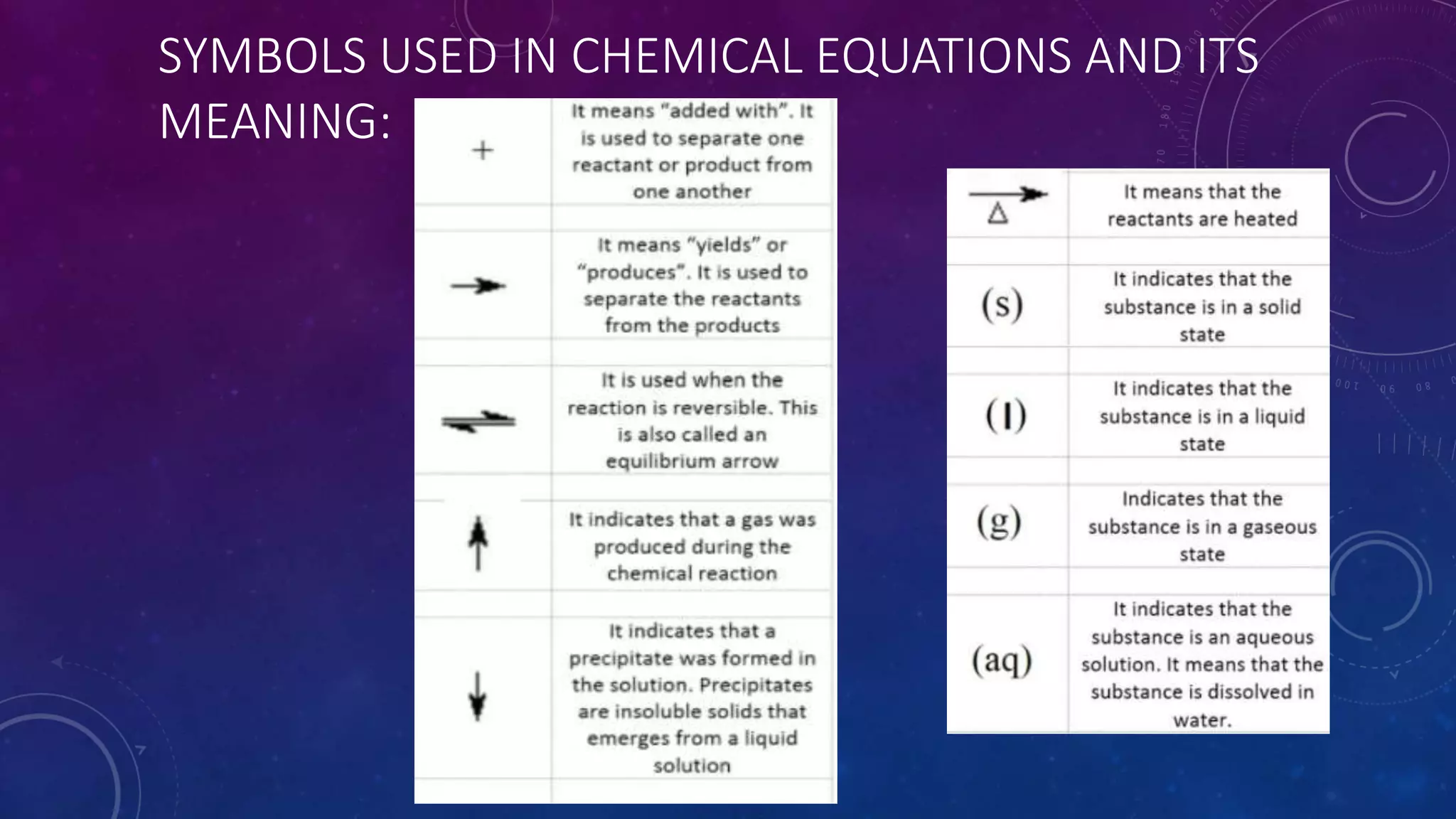



Chemical equations use symbols to represent reactants and products, with subscripts showing the quantity of each element or compound involved in a chemical reaction. Balancing chemical equations ensures the same number and type of atoms are on both sides of the reaction arrow. Stoichiometry uses the balanced chemical equation to calculate quantities in chemical reactions.




