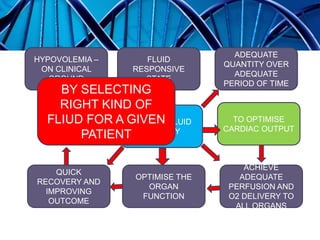
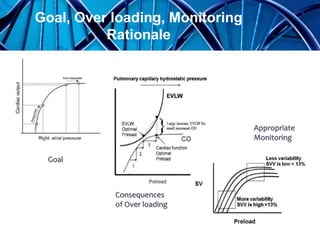
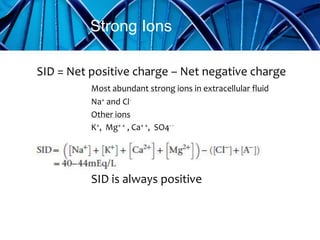
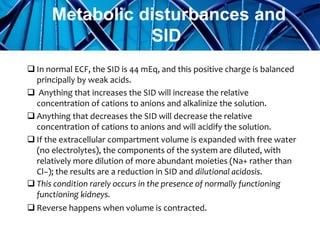
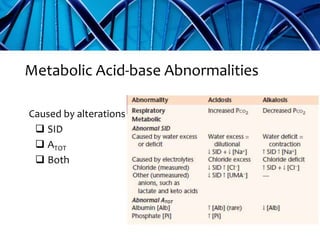
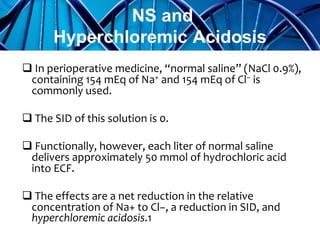
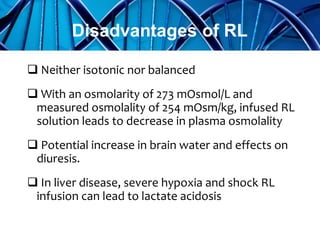
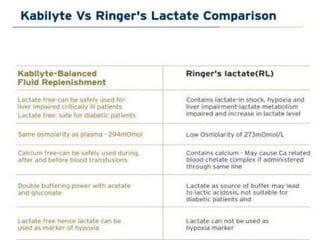
Balanced crystalloids are emerging as the fluid of choice for resuscitation over normal saline. Normal saline can cause hyperchloremic acidosis due to its high chloride content. Balanced crystalloids more closely mimic the electrolyte composition of plasma. Studies show balanced crystalloids may reduce complications like renal injury and need for renal replacement therapy compared to normal saline in critically ill patients. The optimal fluid choice depends on factors like the patient's clinical condition, electrolyte levels, and volume needed.