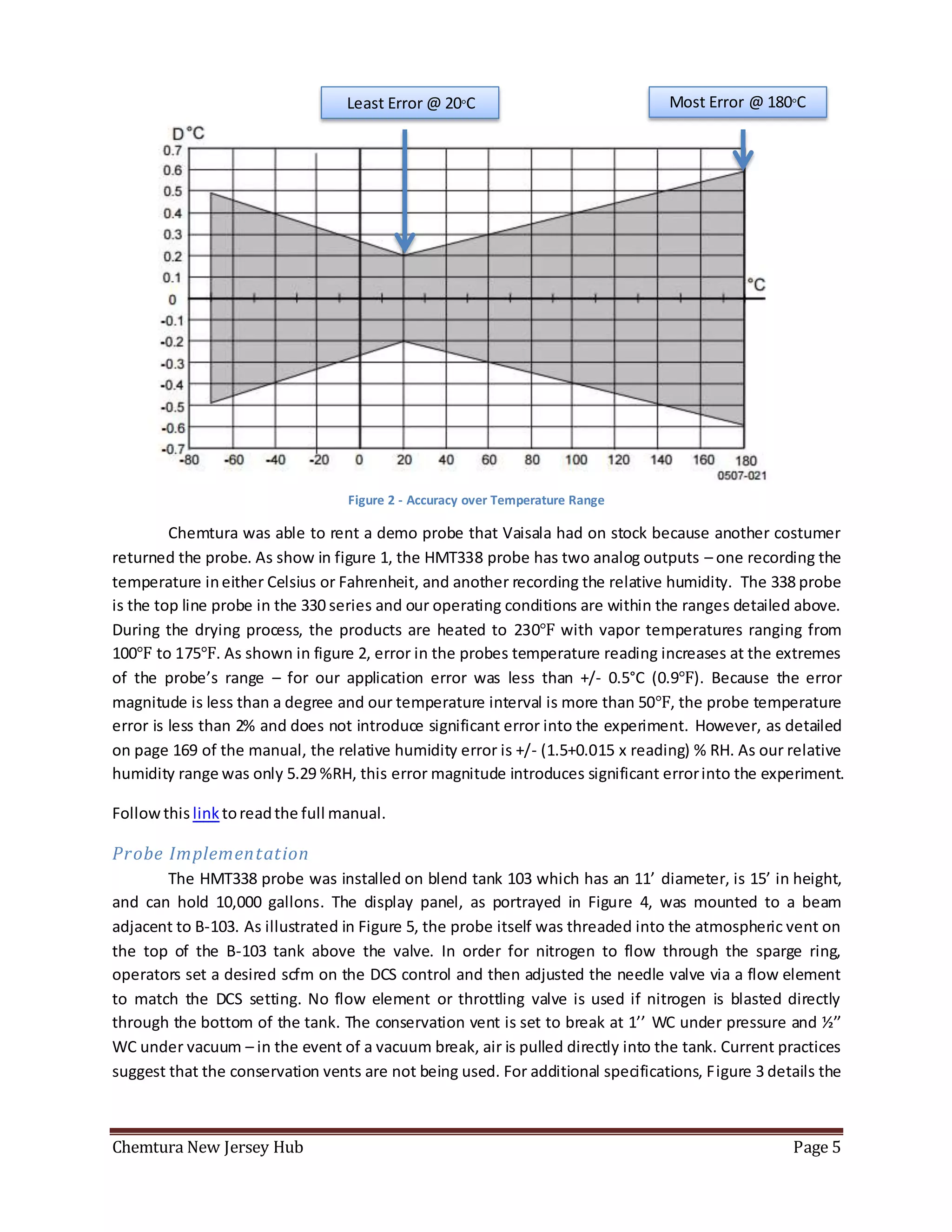
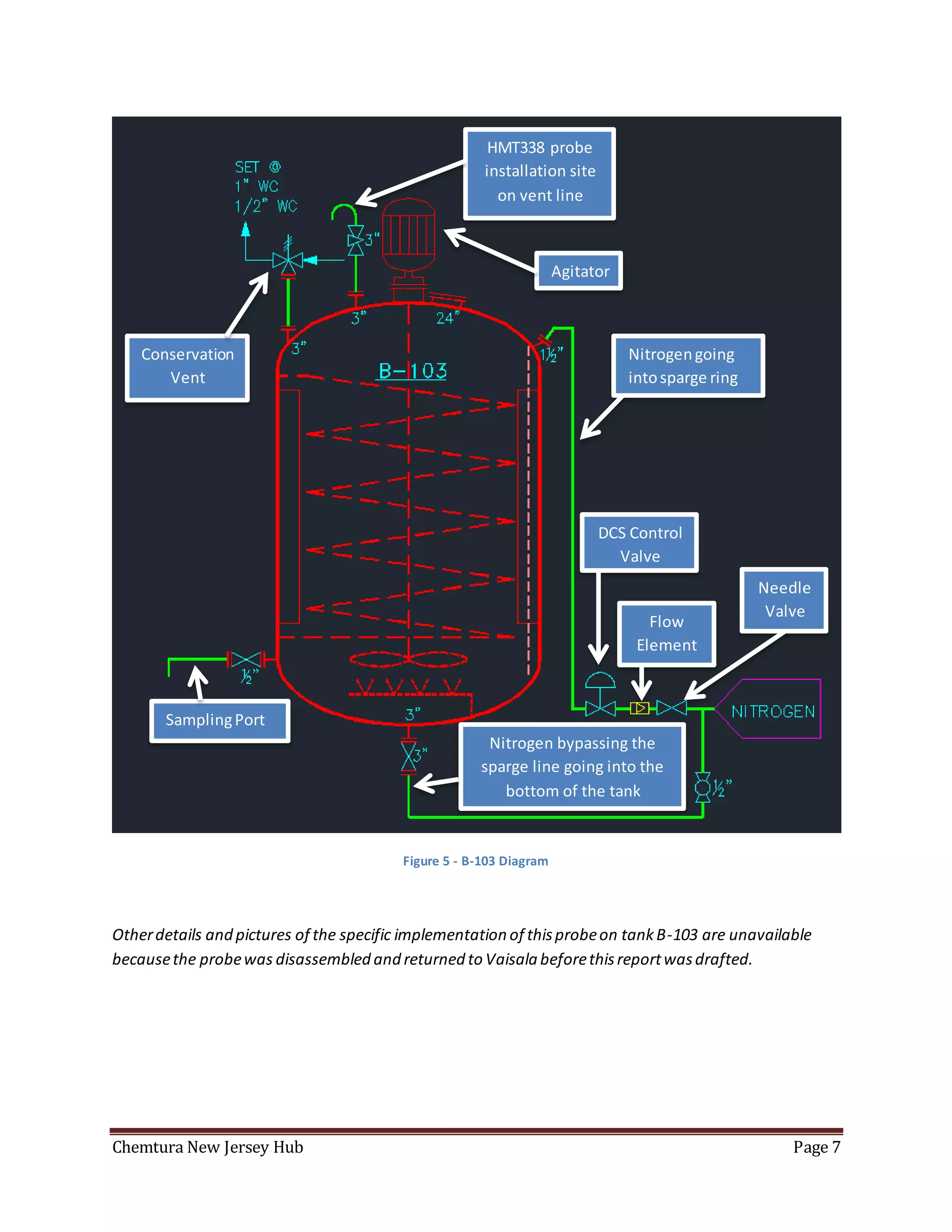
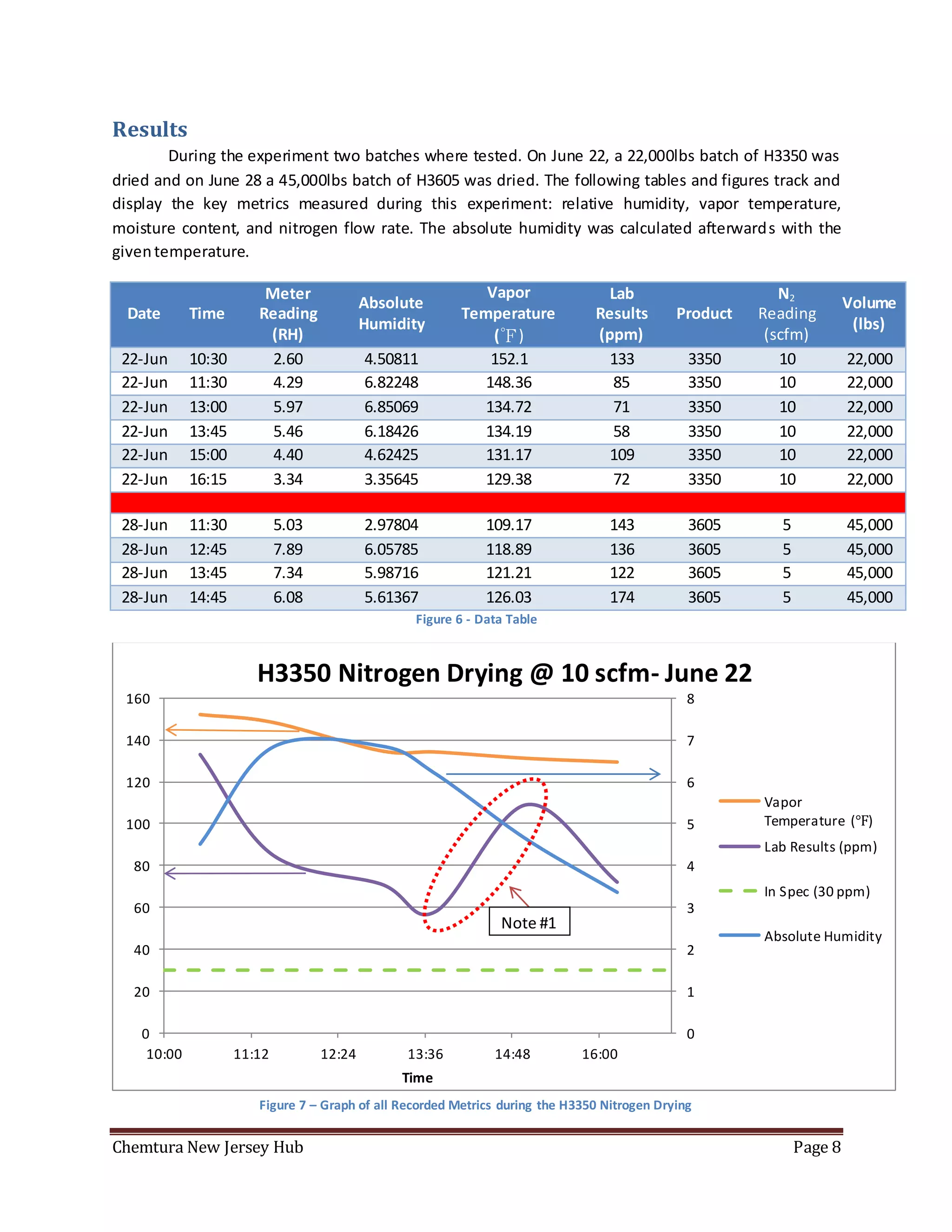
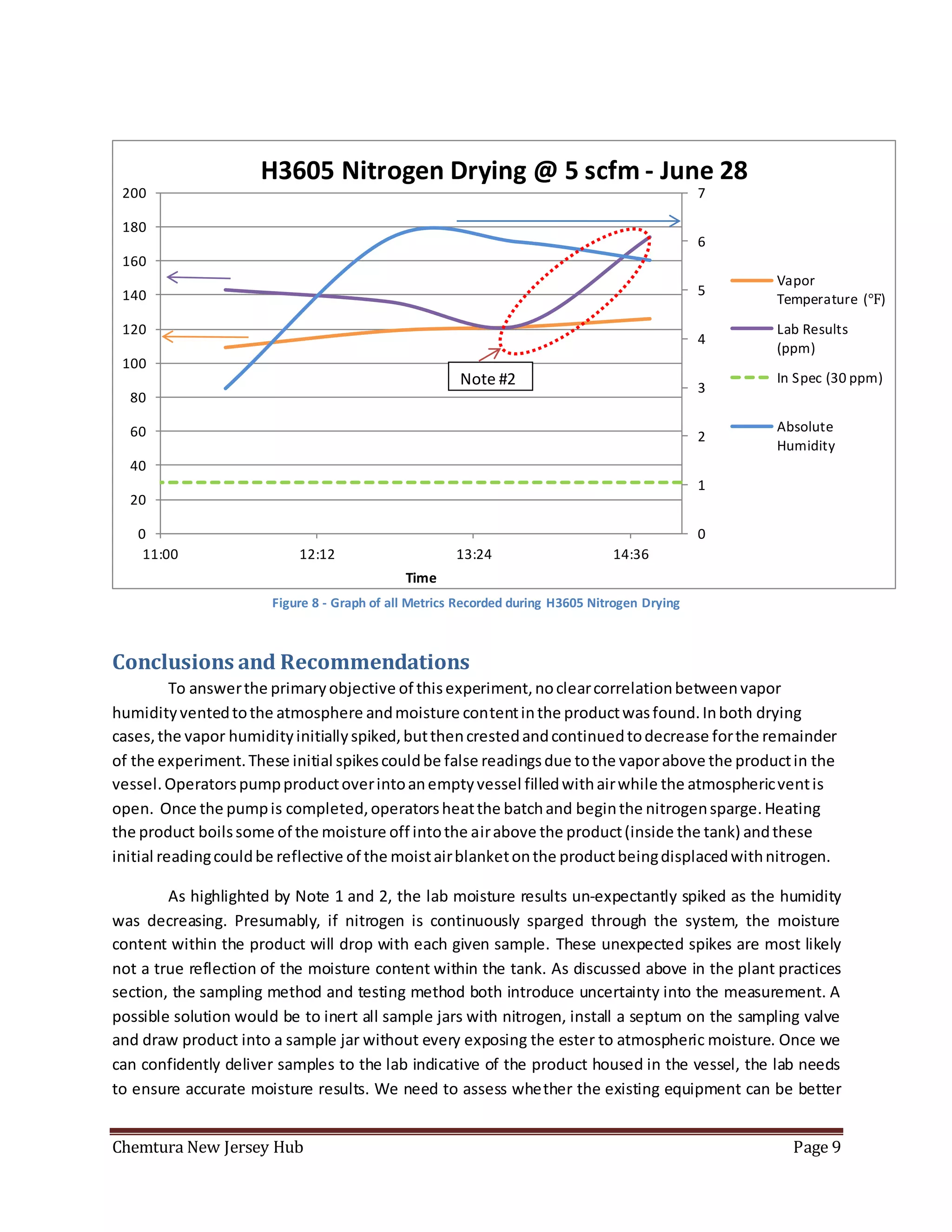
The document summarizes an experiment using a HMT338 humidity probe to attempt to correlate vapor humidity readings from nitrogen drying of specialty ester batches with product moisture content measurements. No clear correlation was found due to confounding factors like sample exposure to atmosphere introducing moisture and humidity-dependent lab testing methods. Recommendations include investigating inert sampling methods, locking down tanks on weekends to minimize nitrogen use, and purchasing more reliable moisture testing equipment.