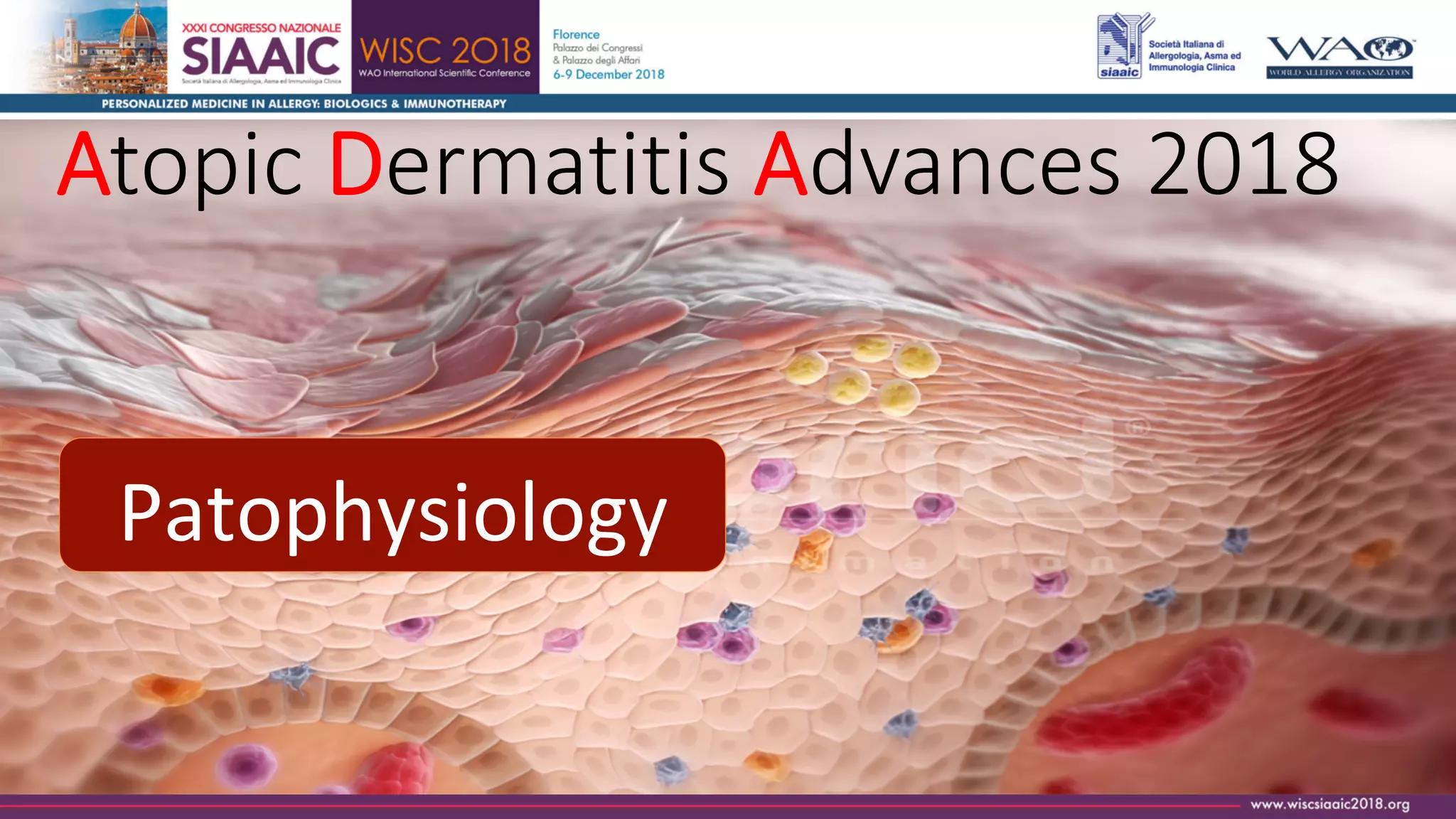
This document summarizes information about atopic dermatitis presented by José Antonio Ortega Martell, M.D. at Atopic Dermatitis Advances 2018. It discusses the pathophysiology of atopic dermatitis involving skin barrier dysfunction and immune system dysregulation. It also summarizes new therapeutic options for atopic dermatitis including the PDE4 inhibitor crisaborole and the biological agent dupilumab, which is a monoclonal antibody that targets the IL-4Rα receptor. The document concludes by noting the promising future of personalized treatment for atopic dermatitis using biological agents and biomarkers to target specific disease endotypes.