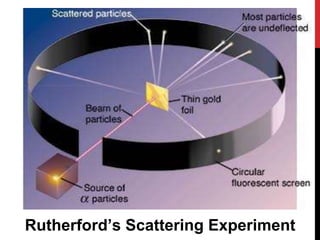
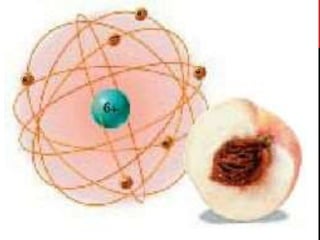
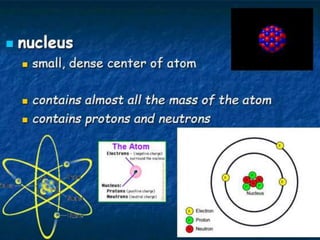
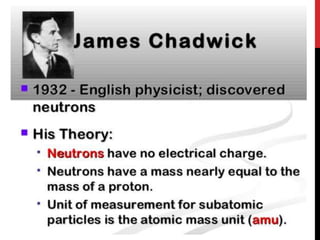
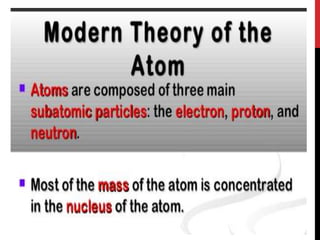
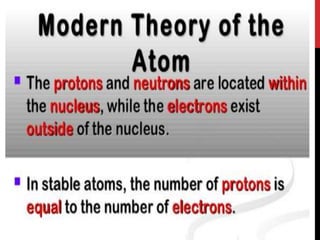
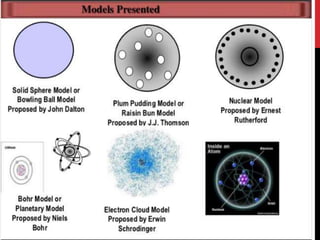
Chemistry began with Aristotle's idea that all matter was made of four elements. Democritus later suggested that matter was made of indivisible particles called atoms. In the 1800s, John Dalton developed the atomic theory stating that all matter is made of atoms that are identical within an element. At the turn of the 20th century, scientists discovered atoms are made of even smaller parts including positively charged protons and negatively charged electrons. Rutherford discovered the nucleus at the center of the atom with electrons orbiting outside. Bohr then proposed electrons orbit in distinct energy levels like planets around the sun. Isotopes with different neutron numbers but the same element were later identified.