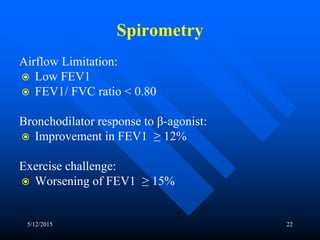
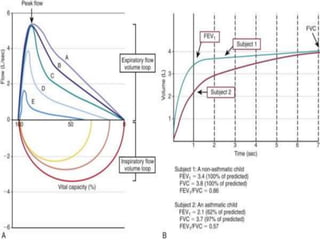
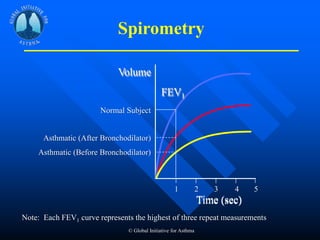
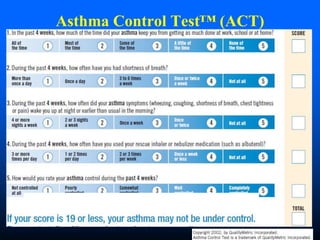
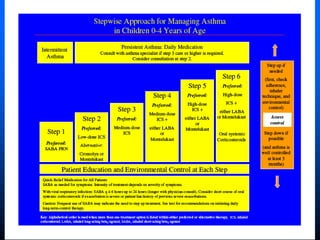
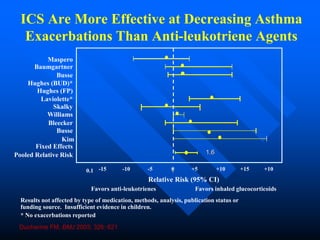
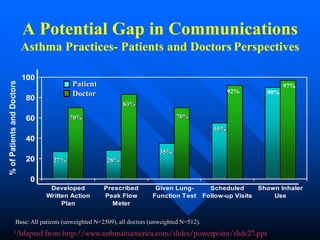
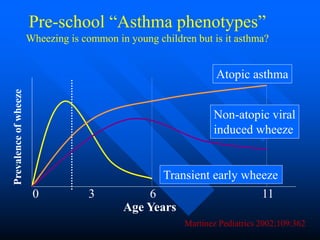
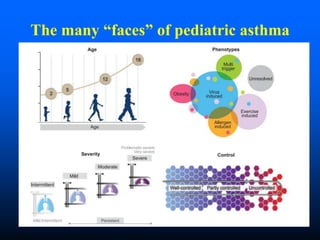
This document discusses asthma in children and provides guidelines for diagnosis and management. It notes that most childhood asthma starts in the preschool years and can be classified into different phenotypes based on risk factors and symptoms. The goals of treatment are to control symptoms and prevent exacerbations. Spirometry can help diagnose and monitor asthma in children over 6 years old, while other tools like peak flow meters and exhaled nitric oxide can help in younger children. Treatment involves a stepwise approach starting with reliever medications and adding controller medications like inhaled corticosteroids based on symptom severity and risk of exacerbations. Close monitoring is important to maintain control and reduce medication doses if possible.







![Pediatric Asthma
Wheezing in Young Children
Transient Early Wheezing
Recurrent wheezing episodes in first years of
life.
Low lung functions, History of Prematurity
Can have severe episodes, requiring
hospitalizations.
Resolves by age 3-5 years.
[ Low Lung function: children improve within a few years and
"outgrow" their asthma ]](https://image.slidesharecdn.com/asthma2015andbeyond-150512152647-lva1-app6892/85/Asthma-2015-and-beyond-8-320.jpg)


![Prevalence of asthma
> 300 million asthmatics in world
[ More than population of Russia ]
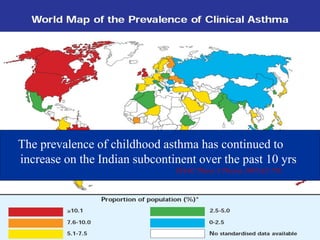
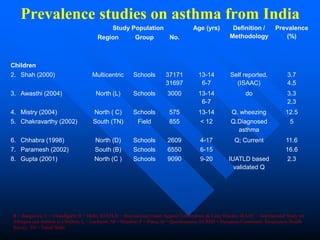
> 30 million asthmatics in India
Significant economic burden
Total asthma costs > Cost of TB & HIV
combined](https://image.slidesharecdn.com/asthma2015andbeyond-150512152647-lva1-app6892/85/Asthma-2015-and-beyond-14-320.jpg)

![Asthma Predictive Index (API)
The Asthma Predictive Index is a useful tool for
predicting asthma in young children
To differentiate “Early wheezers” from
“Persistent wheezers” or children who will
develop asthma
API is the basis for the NHLBI recommendations
for Initiating Long-term Controller Therapy in
Young Children (0-4 years)
95% of - ve by API do not have asthma.Source:Journal of allergy and clinical immunology [0091-6749] Castro Rodriguez, Jose
yr:2010 vol:126](https://image.slidesharecdn.com/asthma2015andbeyond-150512152647-lva1-app6892/85/Asthma-2015-and-beyond-18-320.jpg)