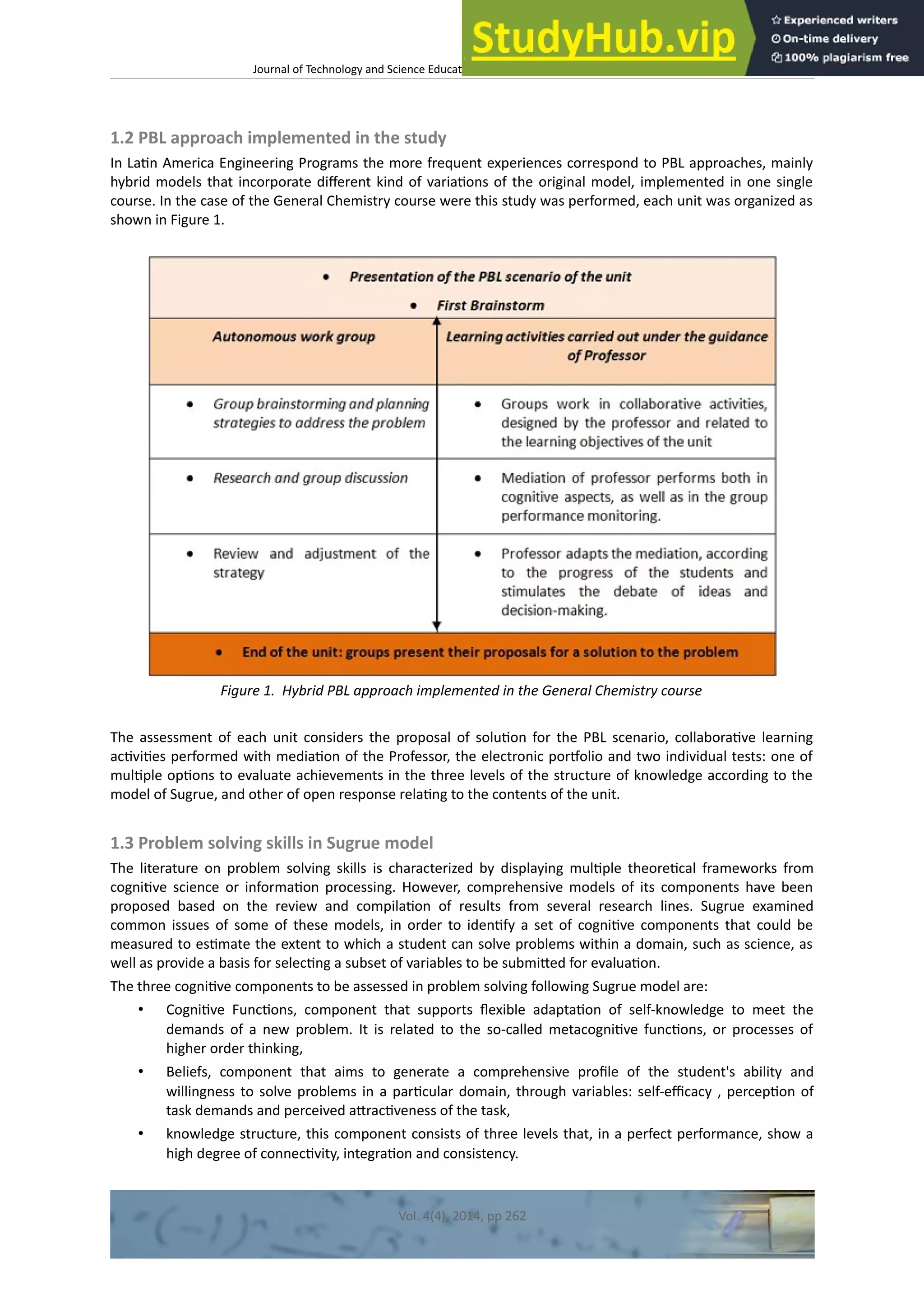
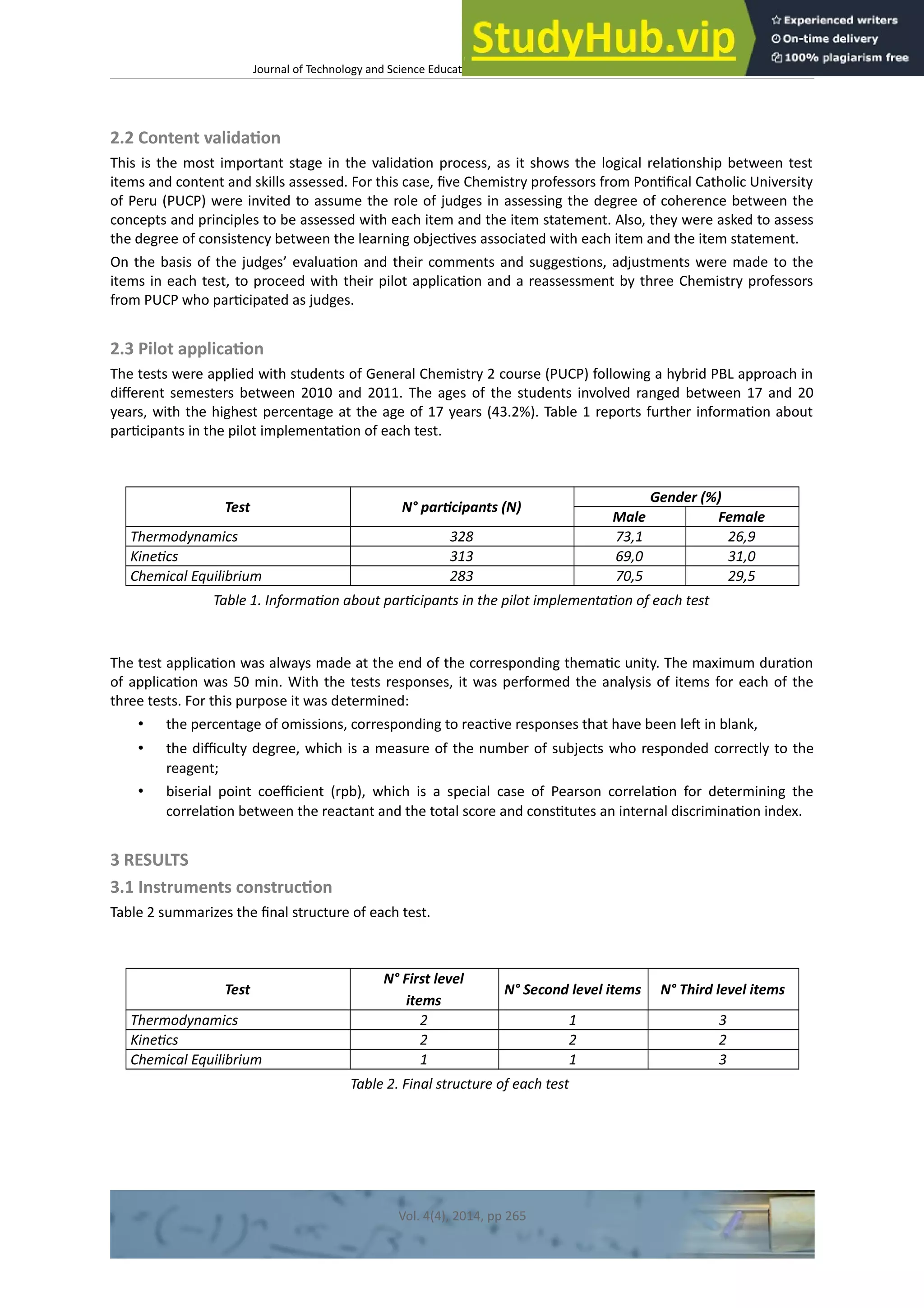
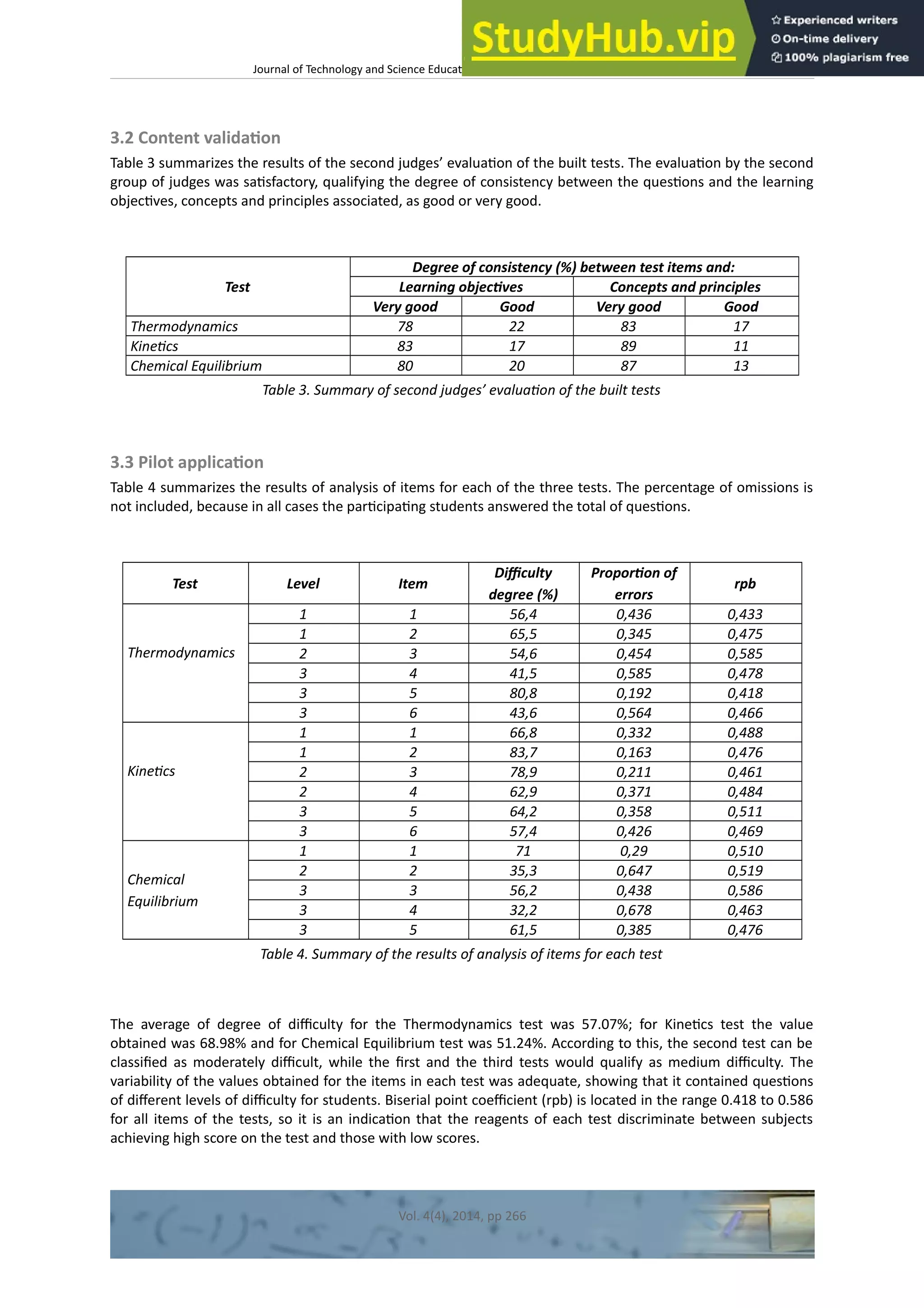
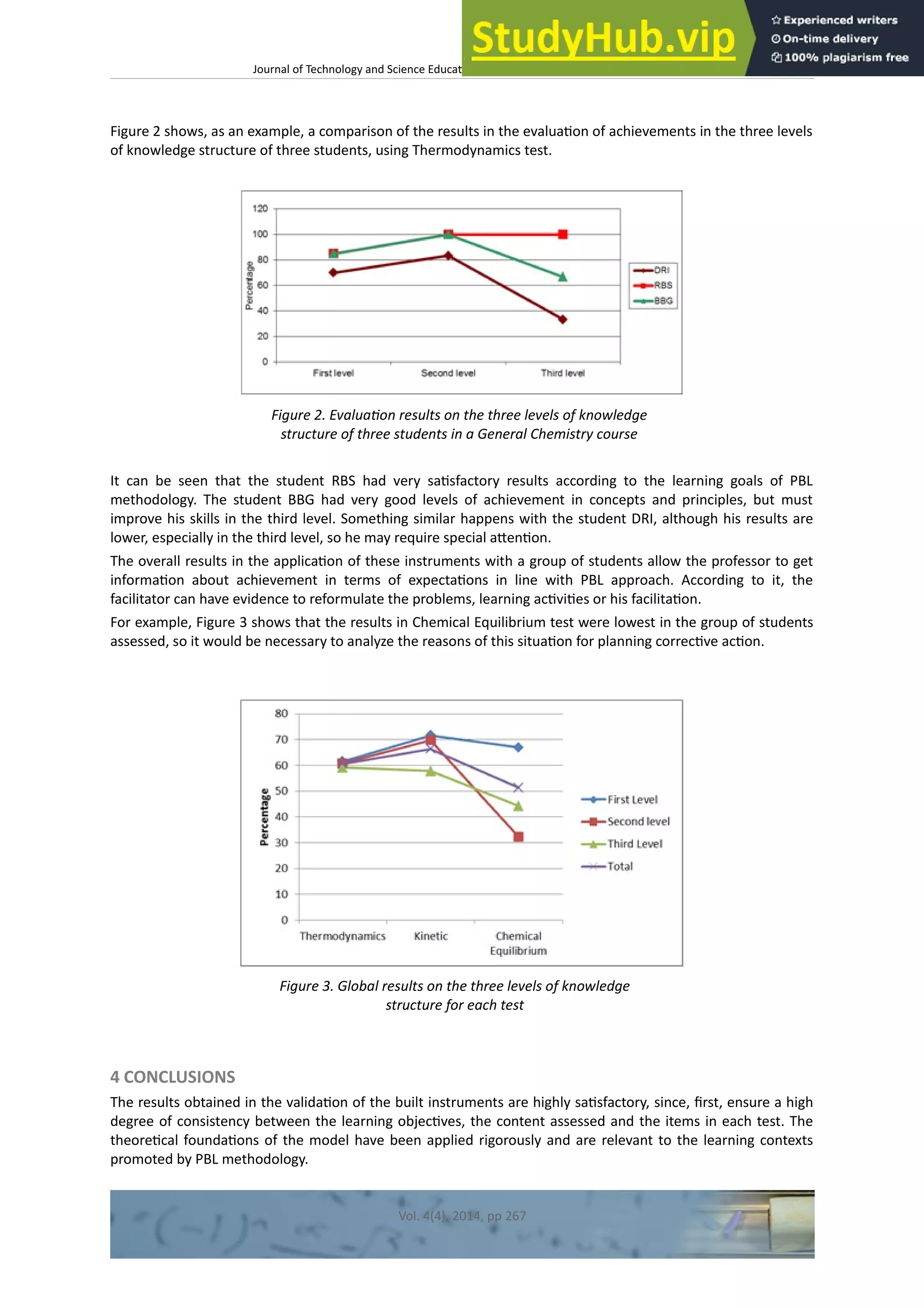
This document discusses the development and validation of tests to assess student achievement in problem-solving skills in a General Chemistry course using a hybrid problem-based learning (PBL) approach. It describes creating tests to evaluate three levels of knowledge structure based on Sugrue's model: concepts, principles, and linking concepts and principles to conditions and procedures. The tests were developed for topics in thermodynamics, kinetics, and chemical equilibrium. The document outlines validating the tests through content validation and a pilot study with Peruvian engineering students. Results from the pilot study were analyzed to evaluate the tests' appropriateness for assessing problem-solving skills to contribute to the learning process and future research.