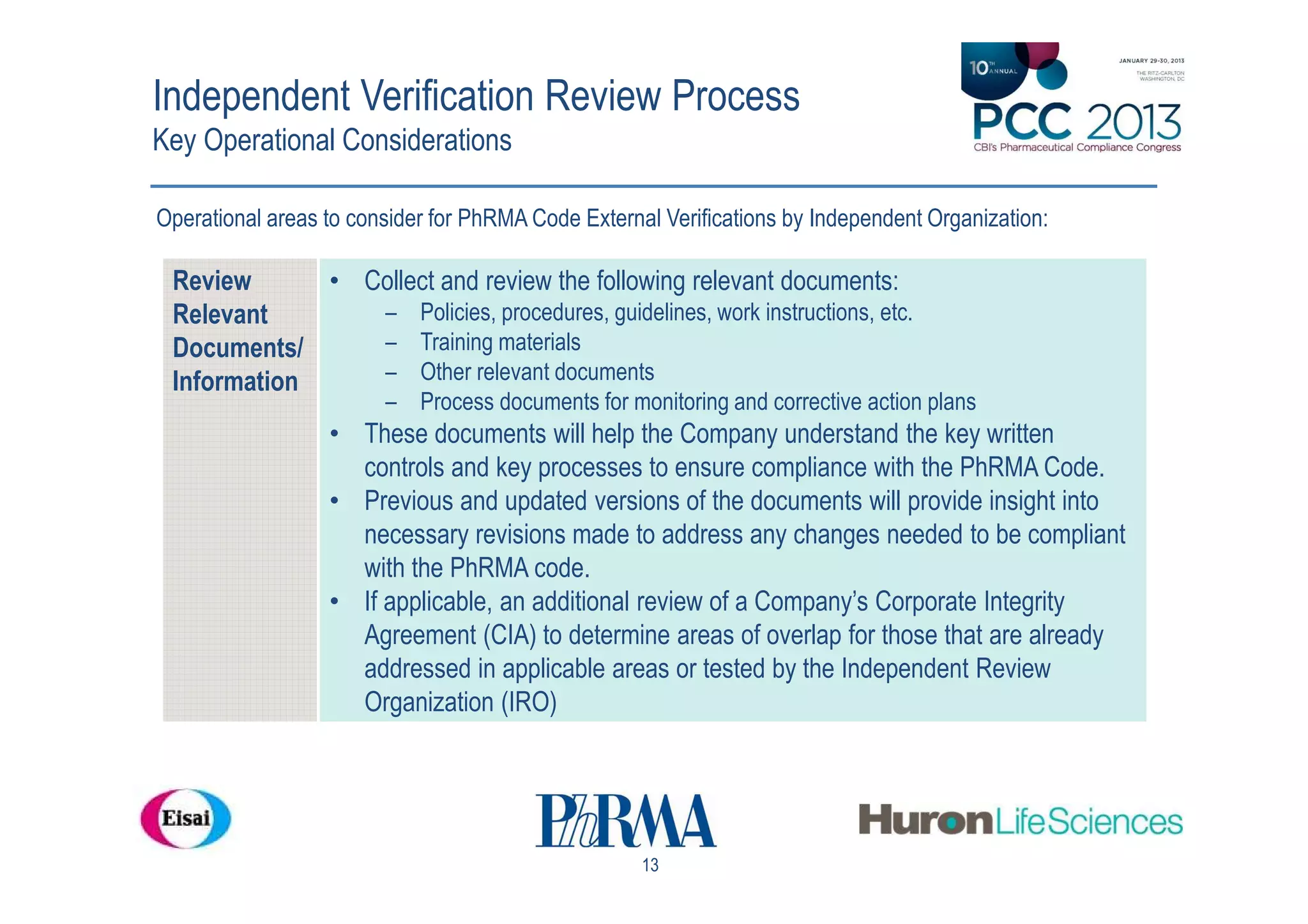
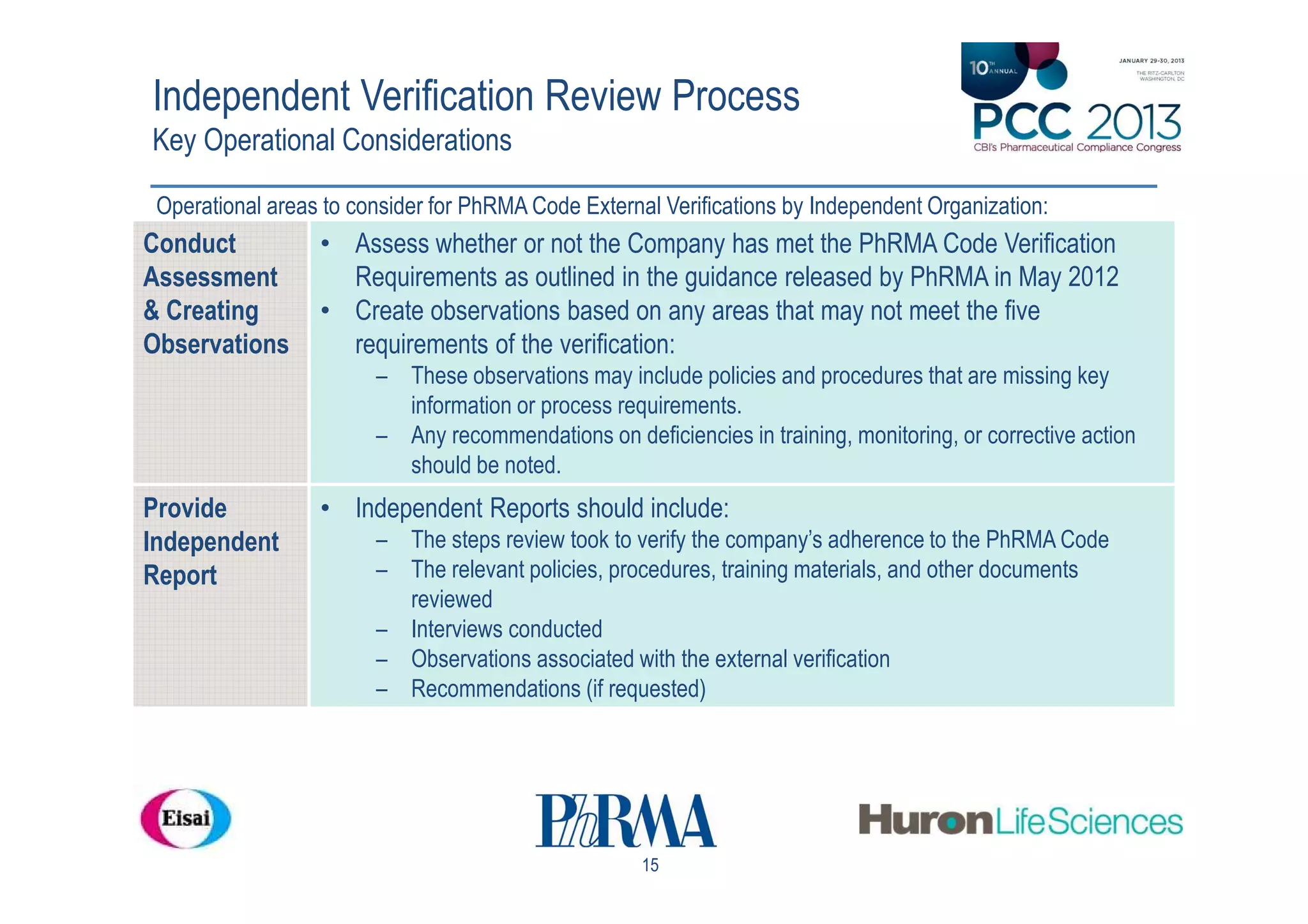
The document outlines a workshop focused on the Pharmaceutical Research and Manufacturers of America (PhRMA) Code regarding adherence in interactions with healthcare professionals. Key points include an overview of the code, guidelines for external verification, and industry insights on compliance and its challenges. It emphasizes the importance of independent verification to ensure that companies meet the code's standards and enhance patient care through ethical interactions.





![Industry Insight on Adherence to the Code
Overlap with State Requirements
• Nevada
– “Does your company use one of the two model codes of conduct [Code of Interactions
with Healthcare Professionals by PhRMA] or Code of Ethics on Interactions with Health
Care Professionals by AdvaMed (for manufacturers or wholesalers of devices or
appliances)] without modification?”
• Massachusetts
– “Our company has adopted a program to routinely train appropriate employees, including,
without limitation, all sales and marketing staff regarding the marketing code of conduct,
as described in 105 C.M.R. 970.000.”
– “Our company has policies and procedures in place for conducting investigations into any
and all non-compliance with 105 C.M.R. 970.000, taking corrective actions in response to
all non-compliance…”
• California
– Annual Declaration of Compliance
6](https://image.slidesharecdn.com/assessingadherencetothephrmacode-130206115851-phpapp01/75/Assessing-Adherence-to-the-PhRMA-Code-7-2048.jpg)