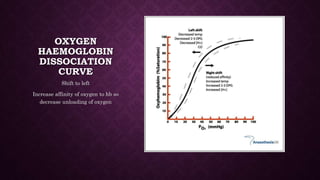
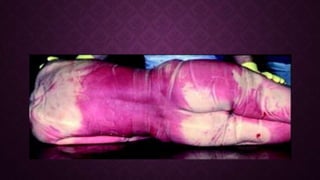
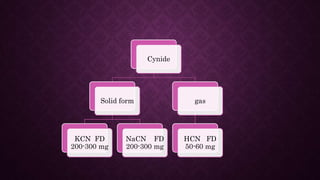
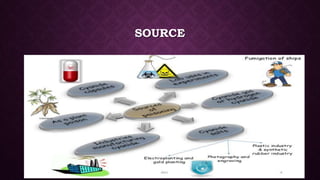
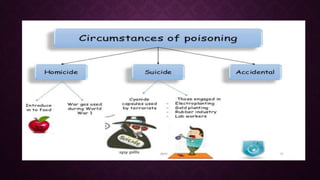
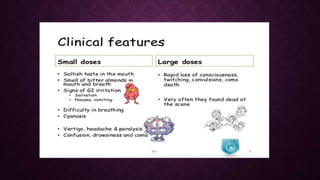
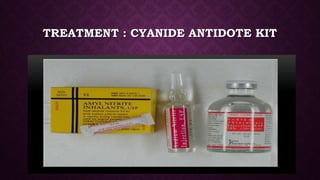
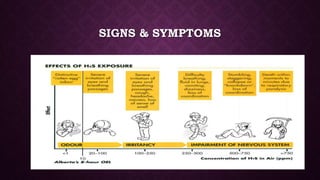
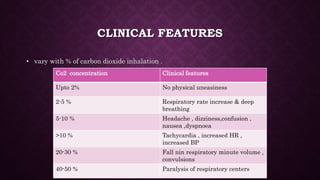
This document discusses various types of asphyxiants including irritants, chemical asphyxiants, simple asphyxiants, and volatile and systemic asphyxiants. It provides detailed information about specific asphyxiants such as carbon monoxide, hydrogen cyanide, hydrogen sulfide, carbon dioxide, and methyl isocyanate. For each one, it describes properties, sources, mechanisms of action, signs and symptoms, treatment, and post-mortem findings. Common features of asphyxiation and approaches to management are also outlined.