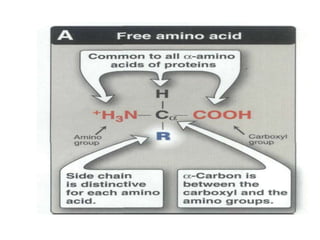
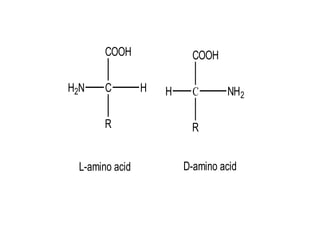
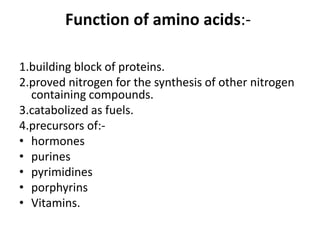
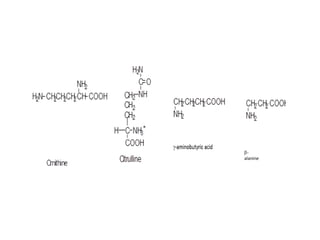
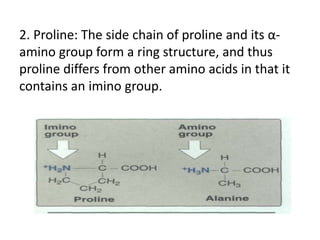
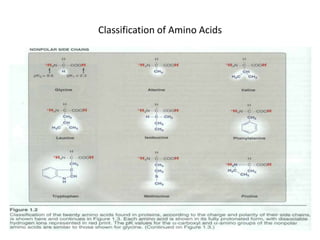
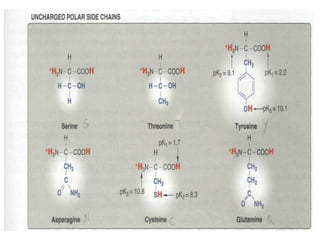
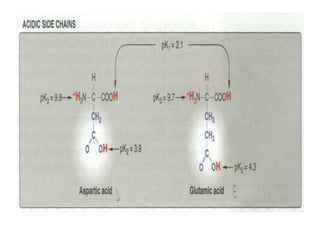
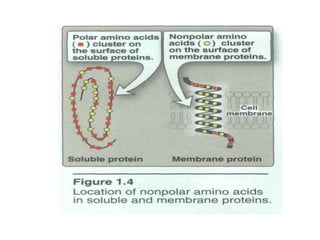
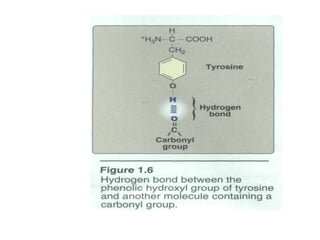
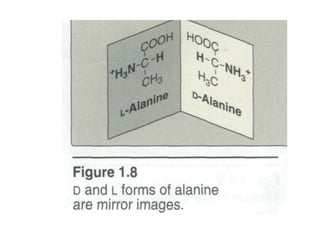
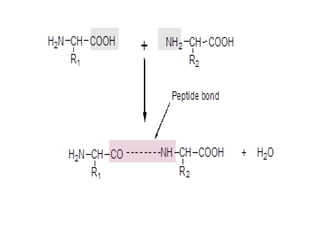
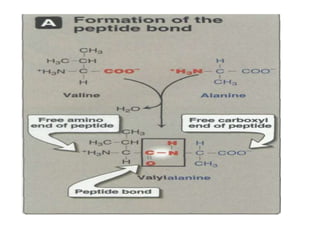
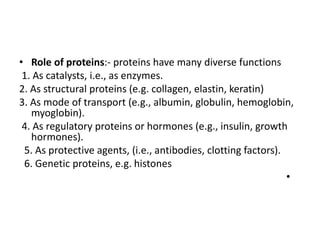
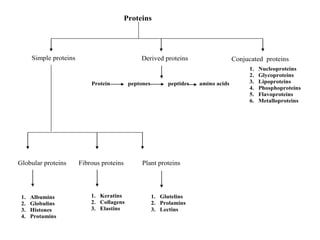
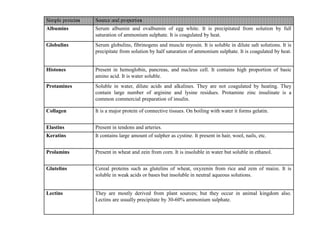
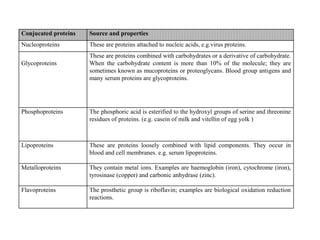
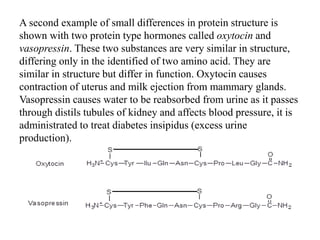
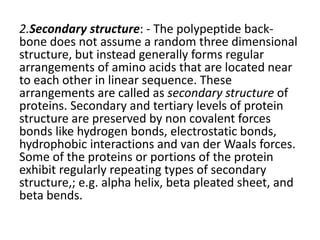
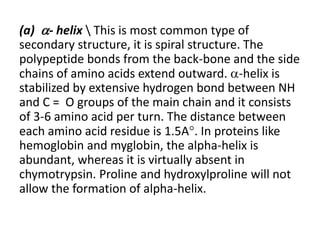
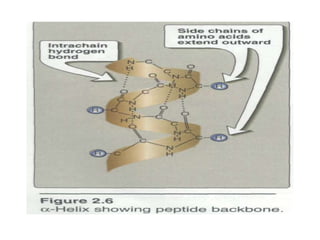
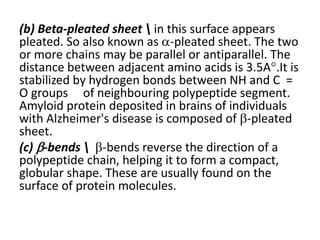
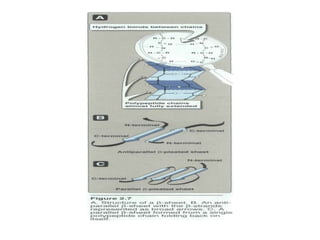
This document discusses amino acids and proteins. It defines amino acids as molecules containing an amino group and a carboxyl group. Amino acids are the building blocks of proteins. Most proteins are made of L-α amino acids joined by peptide bonds. The document classifies amino acids, discusses their structures and functions, and explains how they are joined together to form proteins through different levels of protein structure.