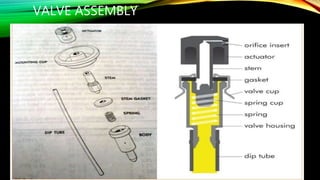
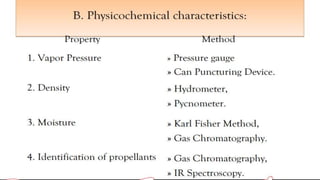
This document discusses aerosol product stability studies. It defines aerosols as pressurized dosage forms containing therapeutic ingredients that emit fine dispersions when actuated. The key components of aerosols are propellants, containers, valves, and product concentrates. Quality control tests examine properties of the propellants and performance of the aerosol, including spray pattern, valve discharge rate, dosage, contents, foam stability, and particle size. Biological testing evaluates therapeutic activity and toxicity. Aerosols require stringent quality control due to risks but are convenient for patients and physicians.