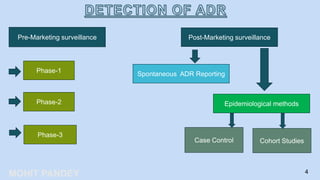
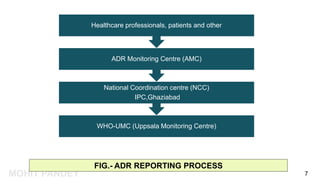
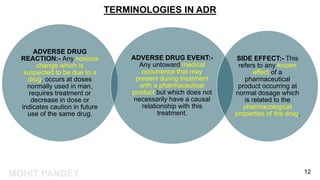
The document is a presentation by Mohit Pandey on adverse drug reactions (ADRs) in the context of pharmacovigilance for his Master of Pharmacy degree. It discusses definitions of ADRs, the importance of adverse event reporting, and methodologies for post-marketing surveillance including cohort and control studies. It also outlines the assessment of seriousness and terminologies related to ADRs, providing guidelines for reporting and evaluation of adverse events.