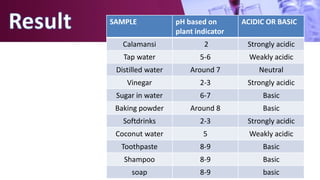
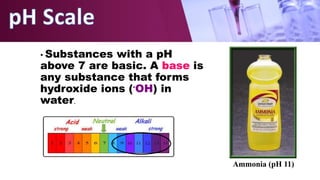
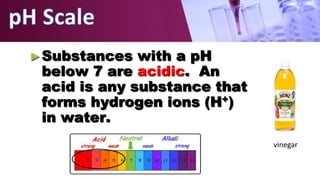
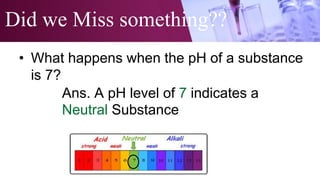
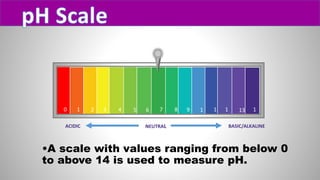
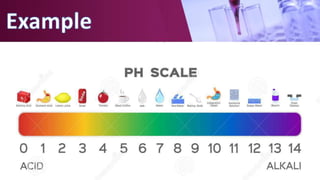
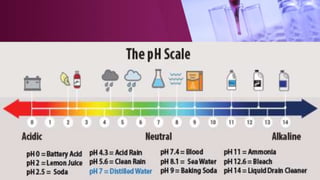
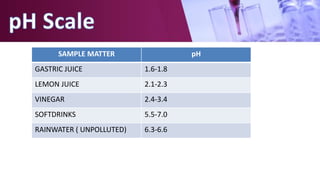
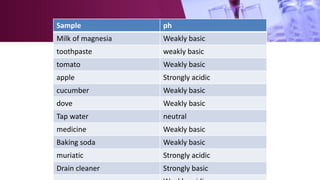
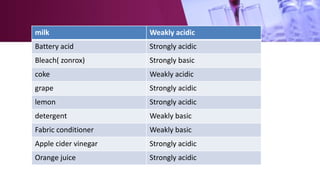
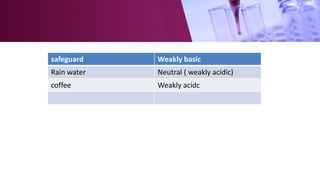
The document discusses pH scales, explaining that pH is a measure of how acidic or basic a solution is, with levels below 7 being acidic and above 7 being basic. It provides examples of the pH of different substances like vinegar, baking soda, and soap. The importance of pH for soil and personal care products is highlighted, noting that plants grow best in certain pH ranges and personal care products aim for specific pH levels to avoid harming the body.