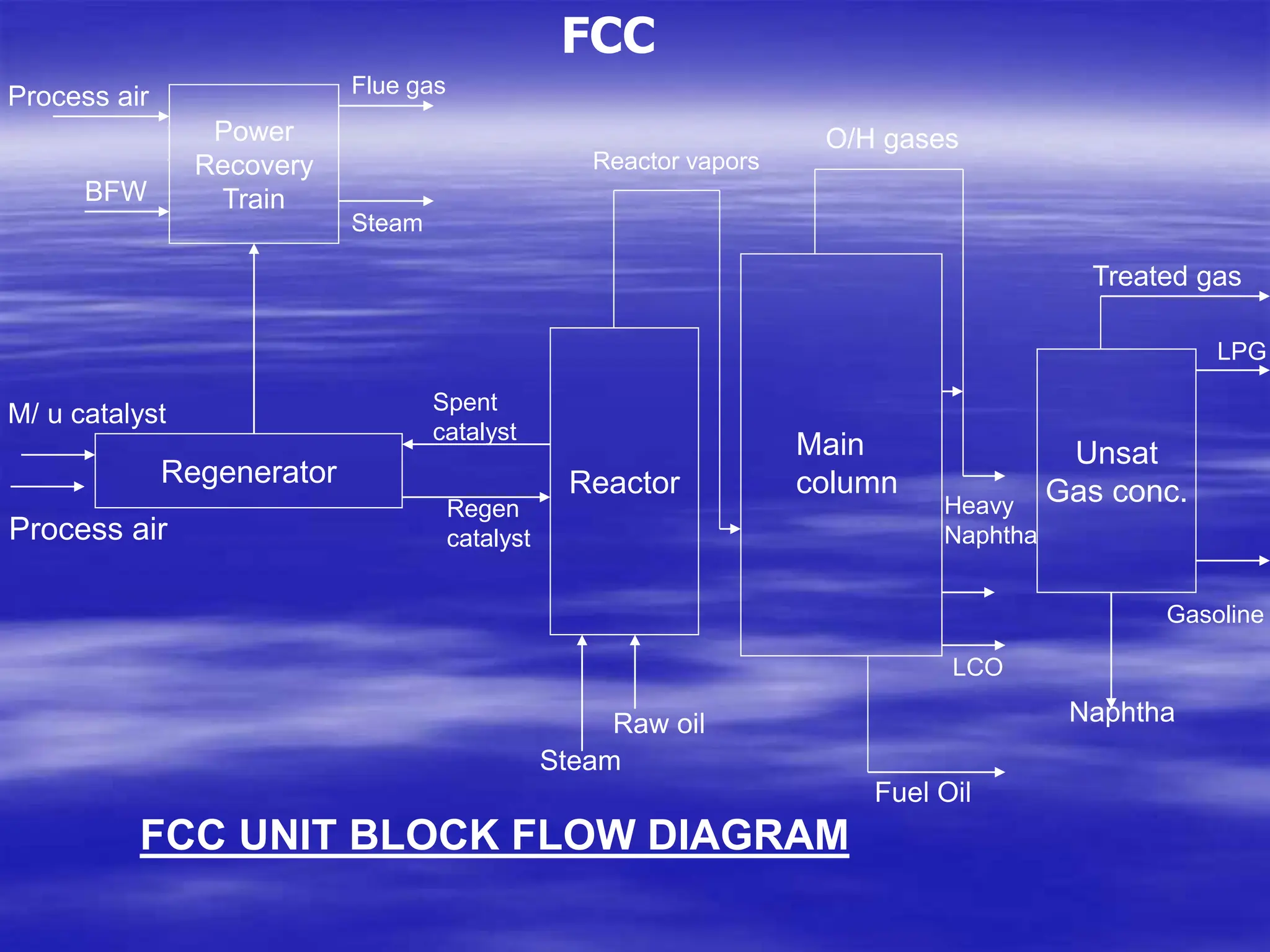
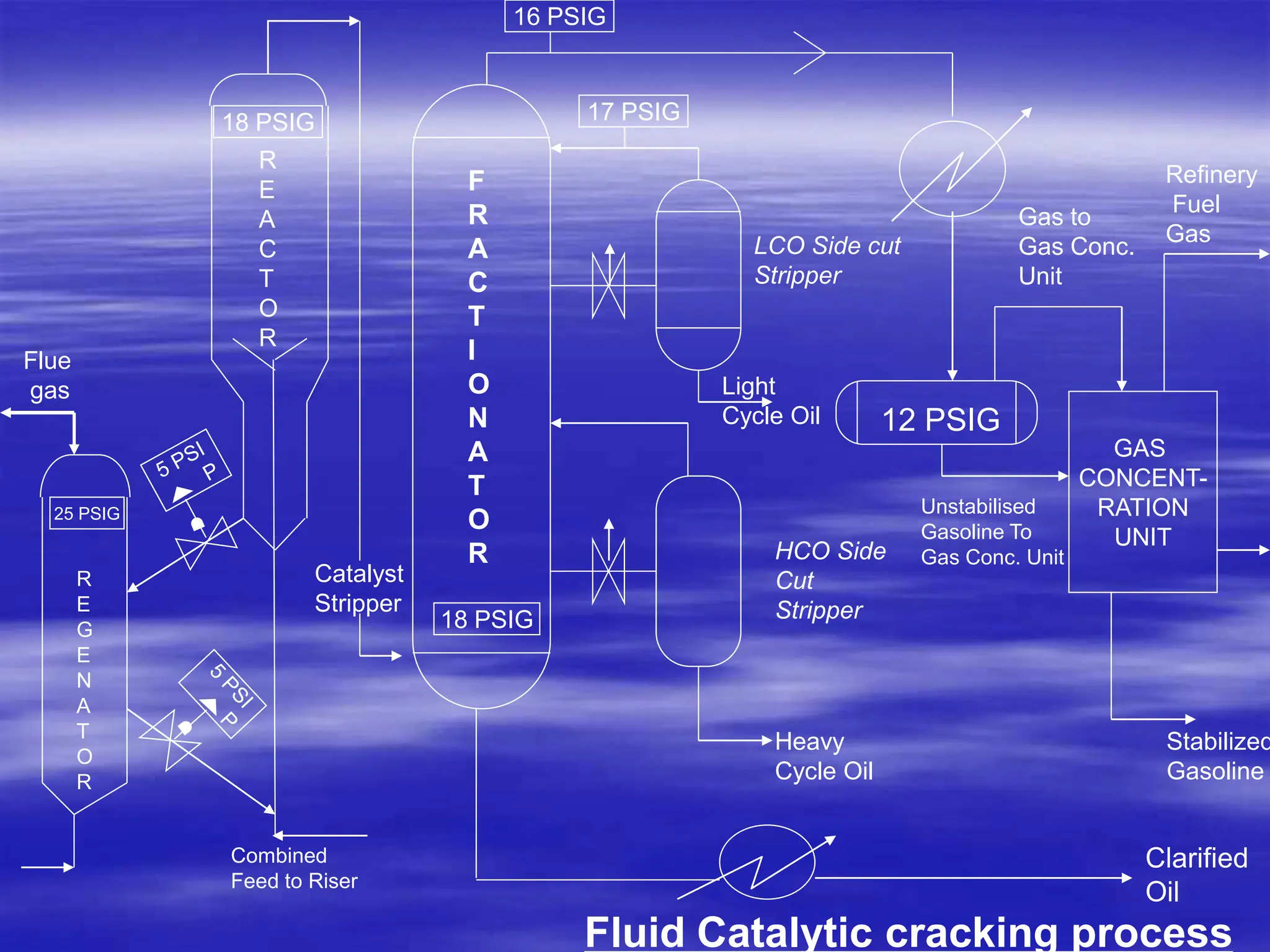
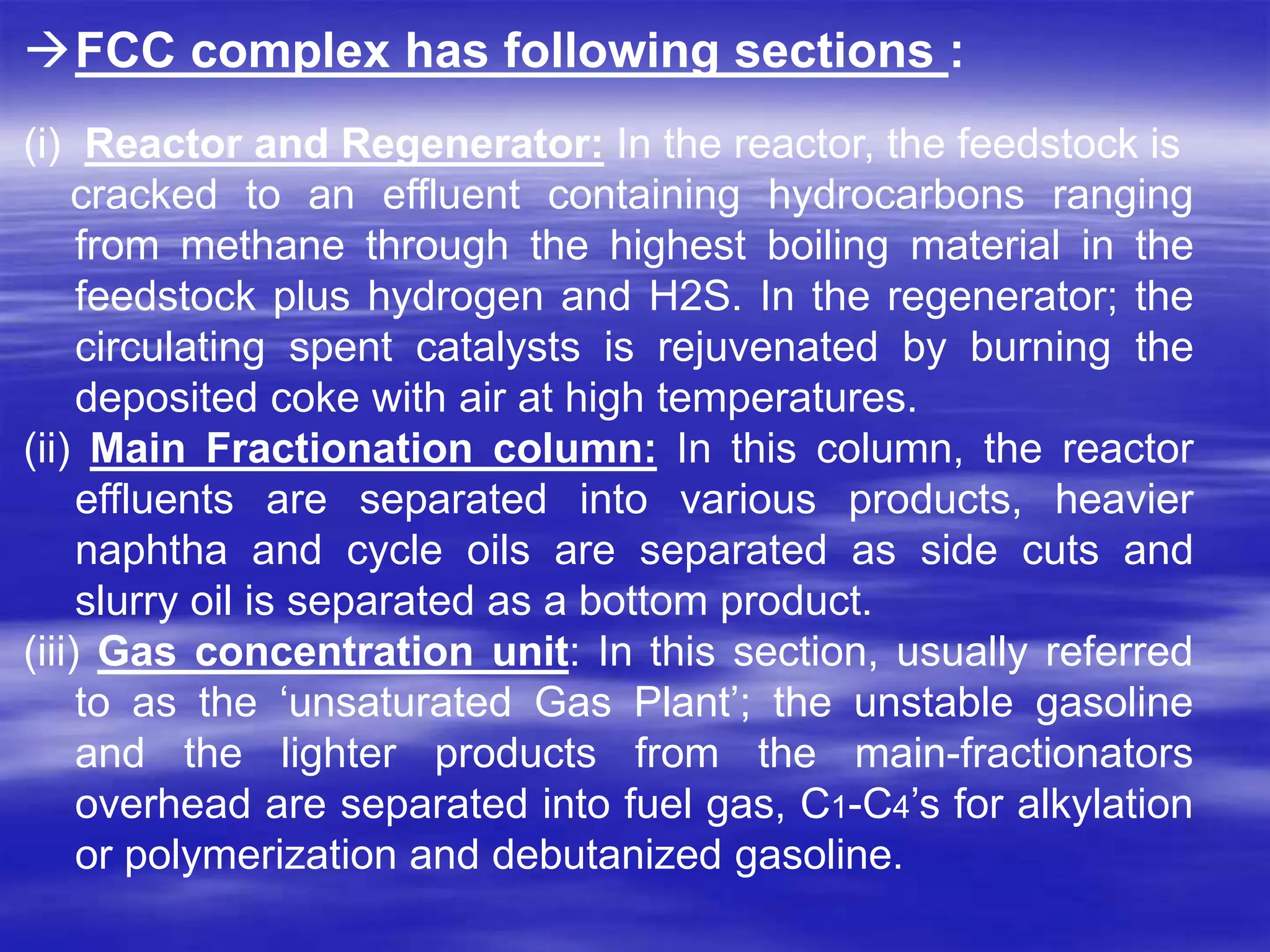
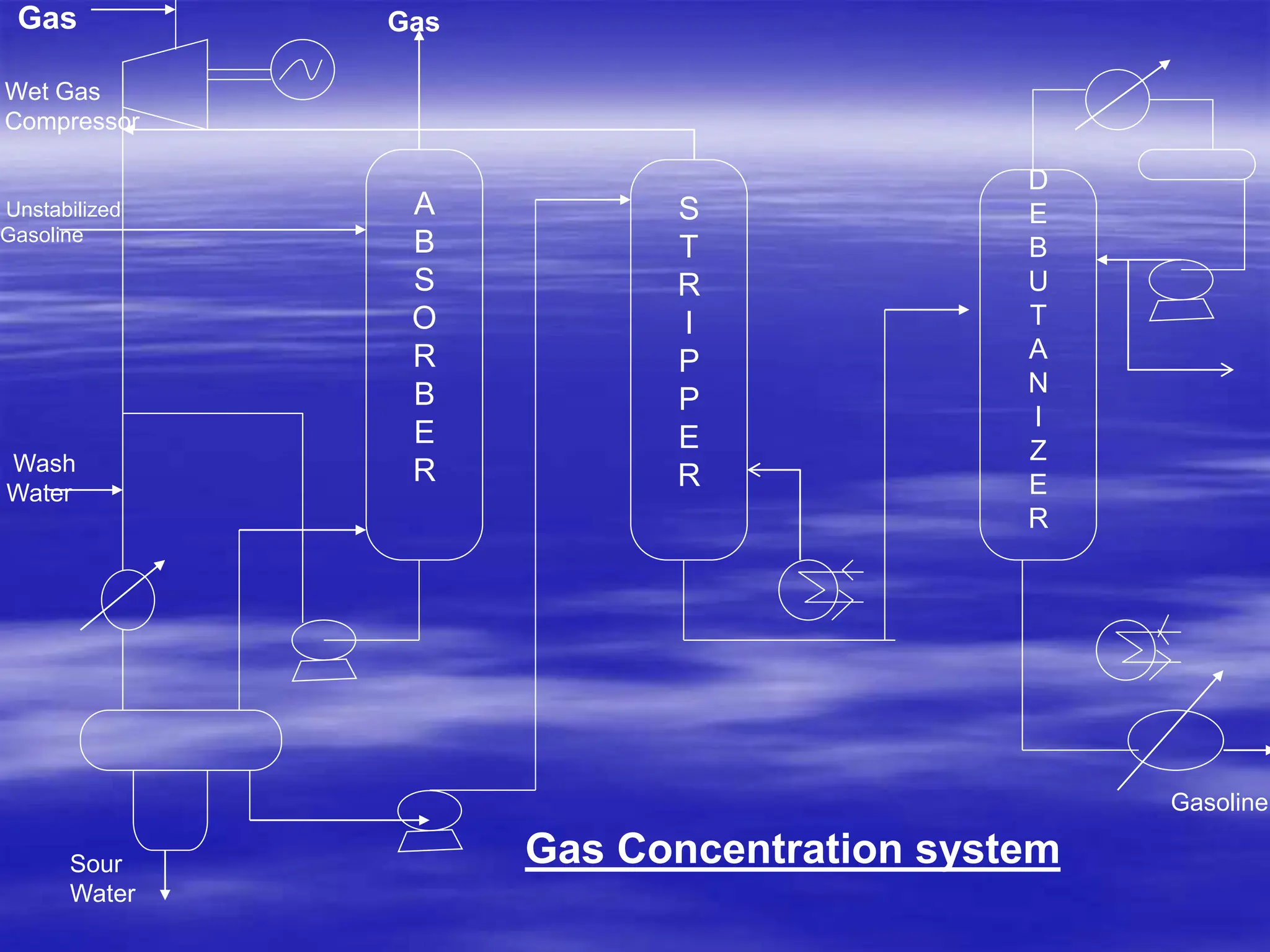
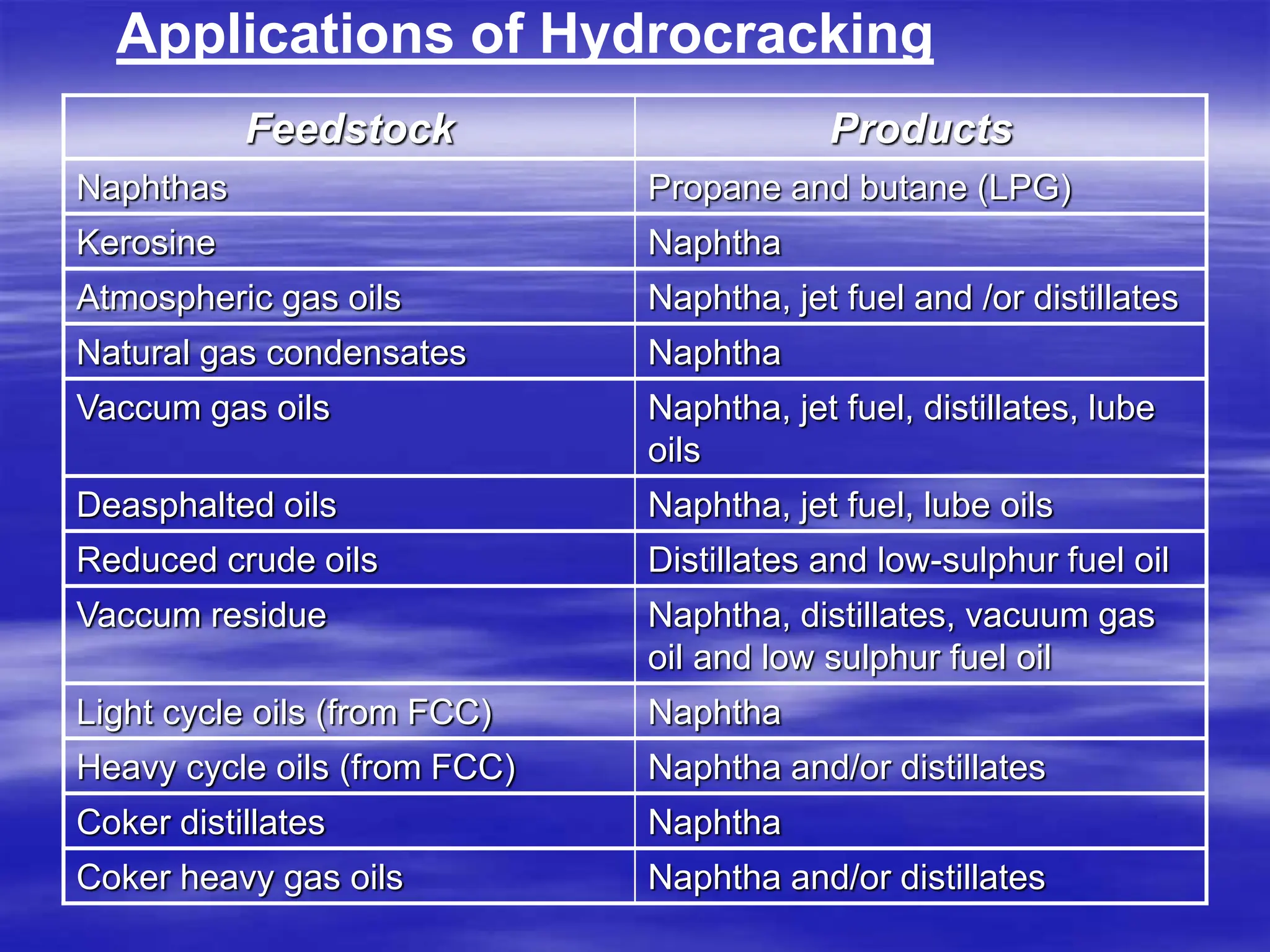
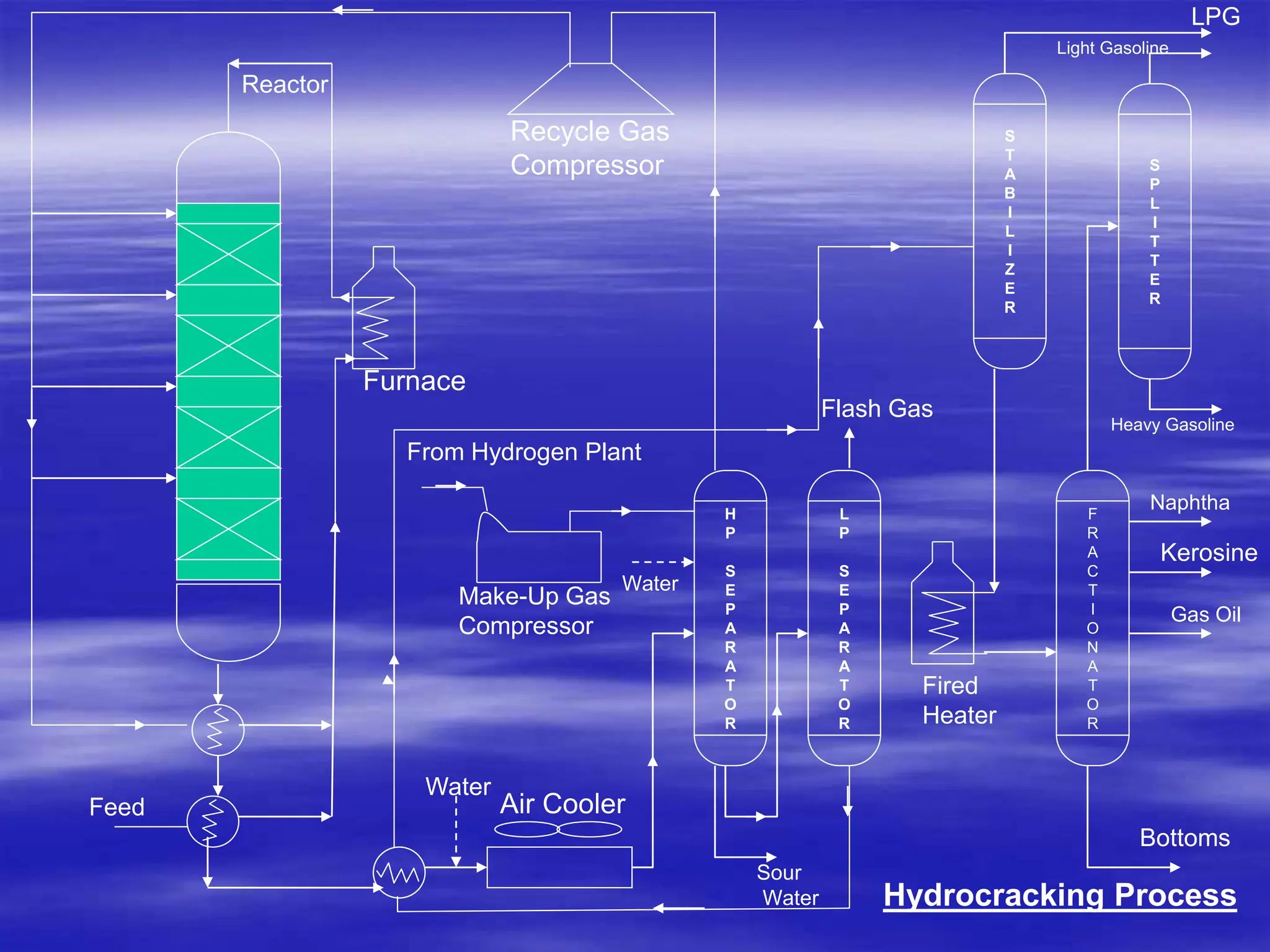
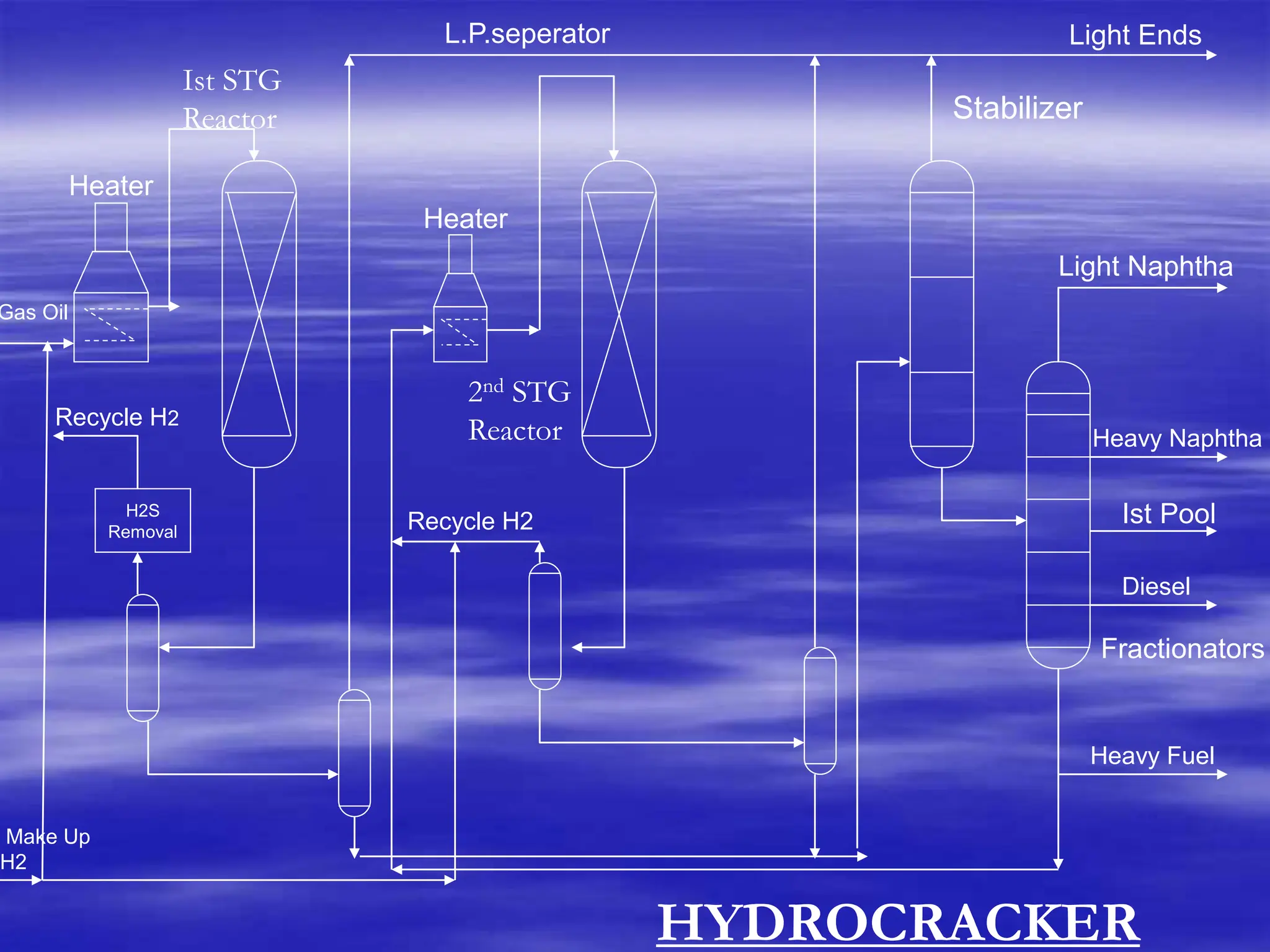
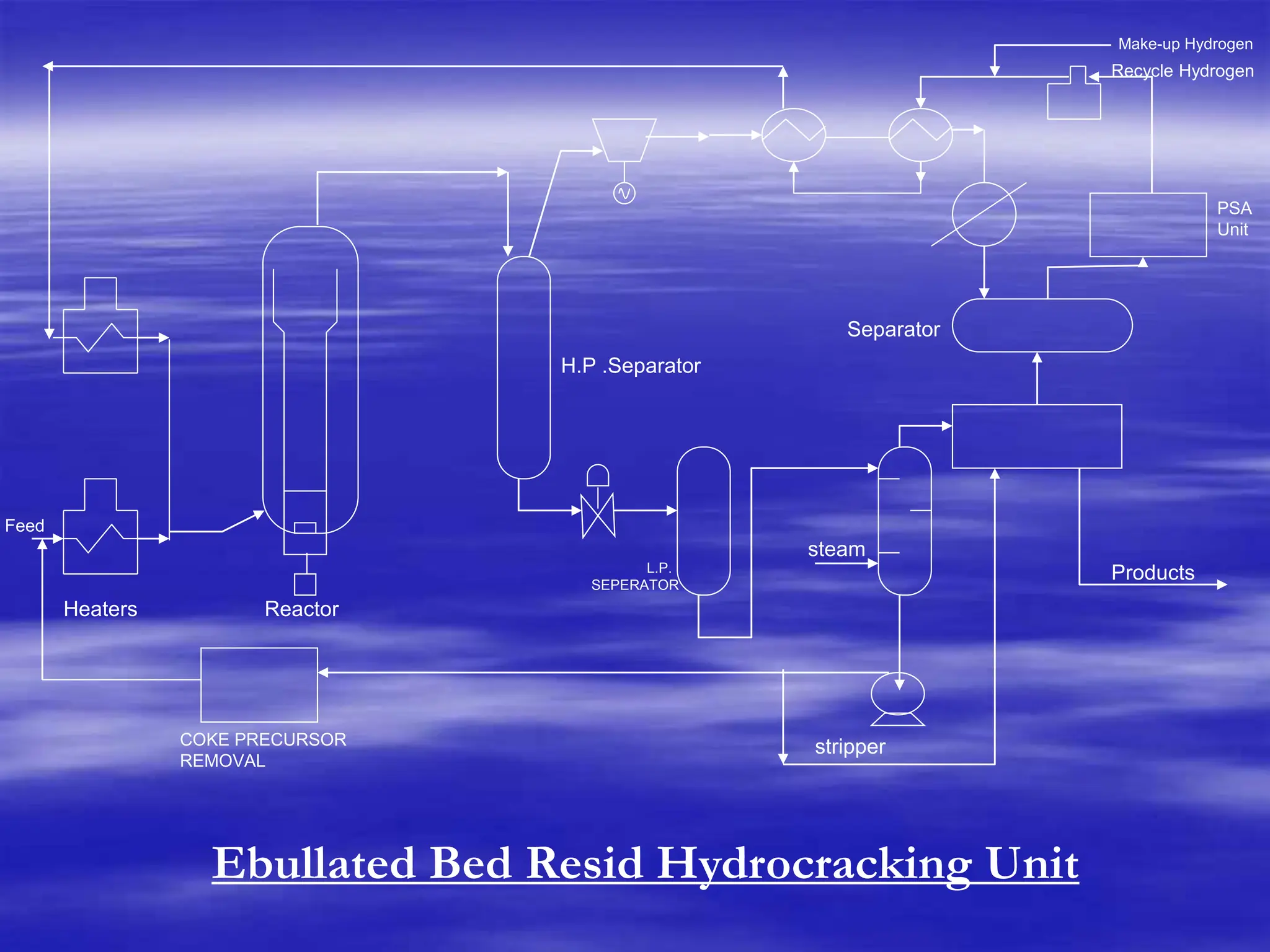
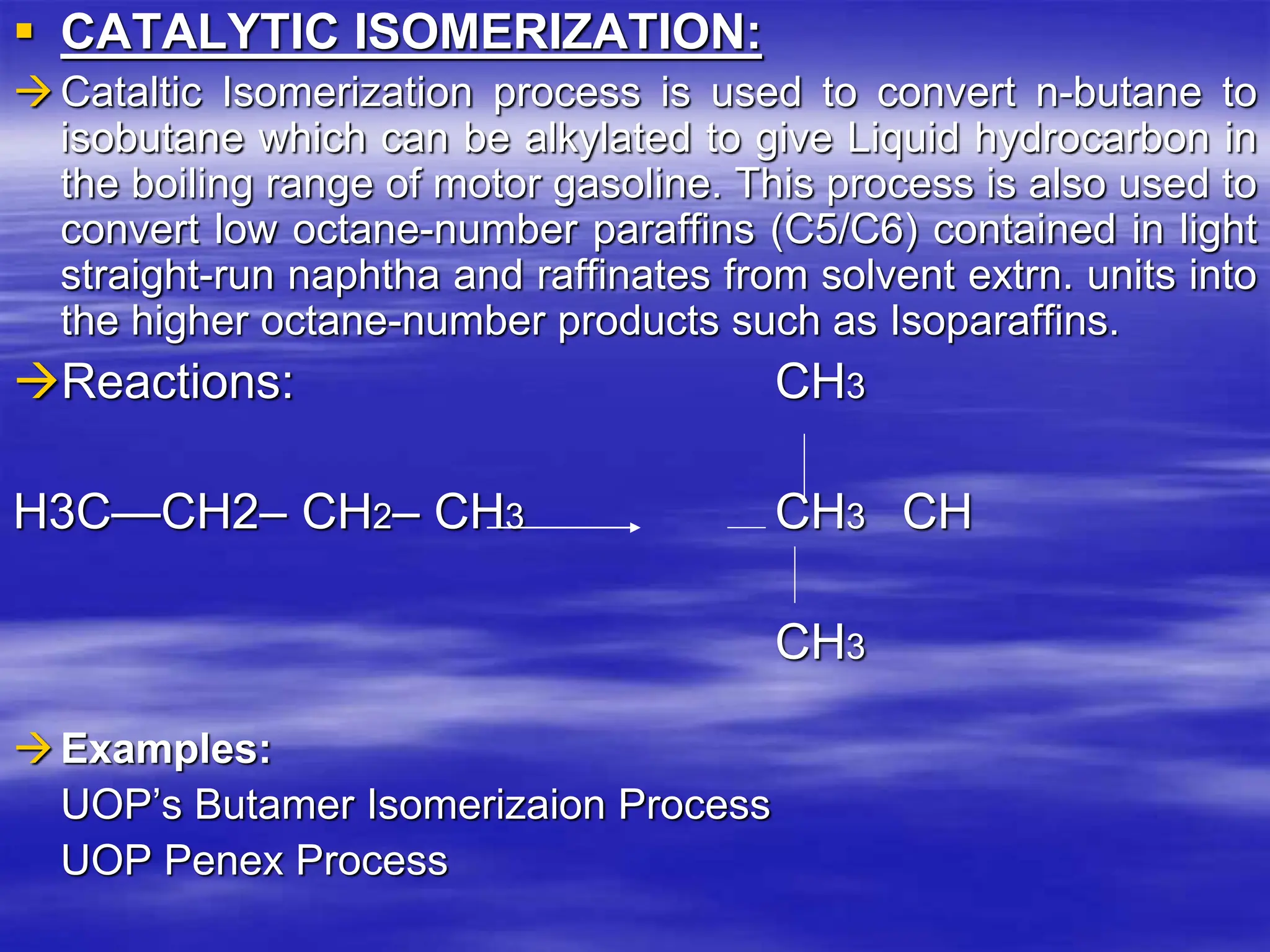
The document provides an overview of various catalytic conversion processes, including fluid catalytic cracking, hydrocracking, isomerization, and catalytic polymerization, used in petroleum refining to transform heavy hydrocarbons into valuable lighter products while removing impurities. It outlines the operational principles, specific applications, and advantages of these processes, emphasizing the versatility and efficiency of technologies like hydrocracking and catalytic cracking. Additionally, it highlights the configurations and energy recovery methods associated with these processes, detailing how they contribute to refining operations.