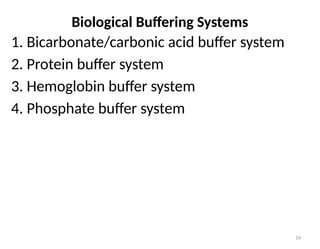
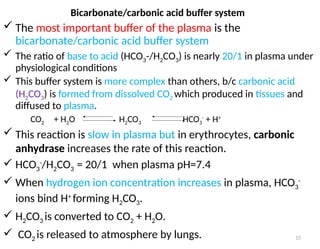
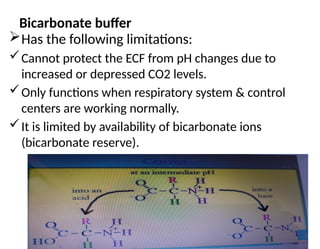
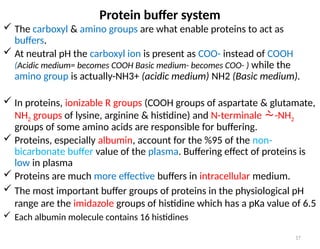
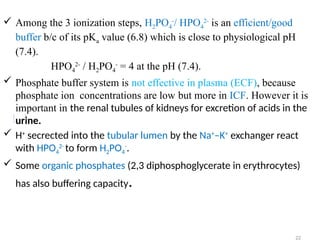
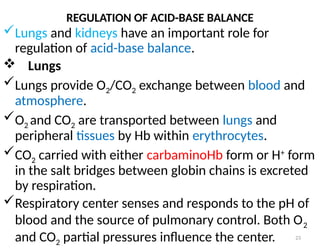
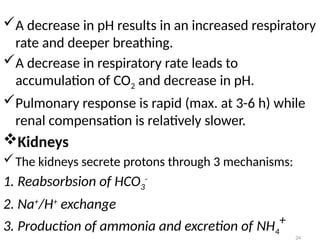
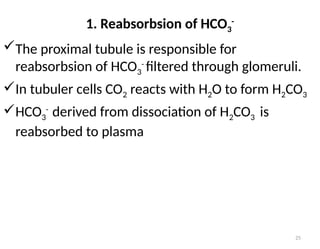
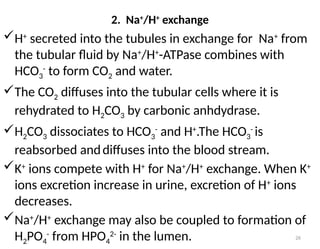
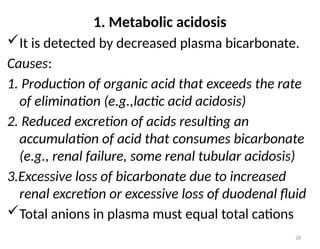
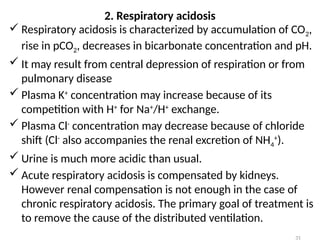
Water is crucial for biological systems, serving as a solvent for chemical reactions, maintaining osmotic concentration, and participating in processes like photosynthesis and temperature regulation. It is also involved in acid-base balance through buffers that help stabilize pH levels within the body, with various systems including bicarbonate, protein, and phosphate buffers. The lungs and kidneys play essential roles in regulating acid-base balance and responding to changes in blood pH.



![8
Acıd-Base Balance and Buffers
The end-products of the catabolism of carbonhydrates, lipids
and proteins are generally acidic molecules in living
organisms.
In metabolic reactions, 22 000 mEq acid (organic acids,
inorganic acids and CO2) is produced per day.
H+
is a direct participant for many reactions, and enzymes.
increased [H+
] can easily alter the charges and functions of
proteins, enzymes, nucleic acids, some hormones and
membranes.
Normal blood pH is 7.35-7.45.
Values <6.8 (Acidosis or acidemia )or > 7.70 are toxic(Alkalosis
or alkalemia ) can result in coma, cardiac failure, and
circulatory collapse.
In living organisms, pH of the body fluids are tightly regulated
by biological buffers and some organs (lungs and kidneys).](https://image.slidesharecdn.com/2-240820133248-d51cfdf8/85/2-water-and-PH-measuring-presentation-pptx-8-320.jpg)
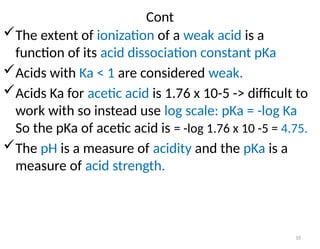
![11
Definition of PH and pka
pH is the (-) logarithm of [H+
]
PH = -log [H+
]
Scale ranges from 1 to 14.
1 means only H+ are present, 14 means no
H+ present.
pOH is the (-) logarithm of [OH-
]
pOH = -log [OH-
]
For water;
[H+]=[OH-]=10-7
M, pH=7 and
pH+pOH=14 (calculated)
• A solution with PH<7 is acidic and
• A solution with PH>7 is basic
Acids are [H+
] donors
Bases are [H+
] acceptors](https://image.slidesharecdn.com/2-240820133248-d51cfdf8/85/2-water-and-PH-measuring-presentation-pptx-11-320.jpg)
![12
Buffers
₋ Buffer is a solution ( or a substance) that has the ability to maintain pH &
bring it back to its optimal value by addition or removal of H+
; Buffer
+ H+
H+
Buffer
₋ Buffer tend to resist changes in pH when small amounts of strong acid
[H+
] or strong base [OH-
] are added.
₋ A buffer system consists of:-
a weak acid (the proton donor) and its conjugate base (weak bases
and their salts).
₋ A mixture of equal concentrations of acetic acid and acetate ion is a buffer
system.
₋ One can soak up excess protons (acid), the other can soak up excess
hydroxide (base).
₋ When a strong acid (HCl) is added:
CH3COO-
+ HCl CH3COOH + Cl-
-
When a strong base (NaOH) is added:
CH3COOH +NaOH CH3COO-
+H2O + Na+](https://image.slidesharecdn.com/2-240820133248-d51cfdf8/85/2-water-and-PH-measuring-presentation-pptx-12-320.jpg)