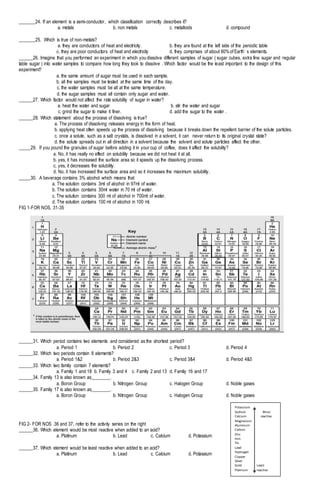
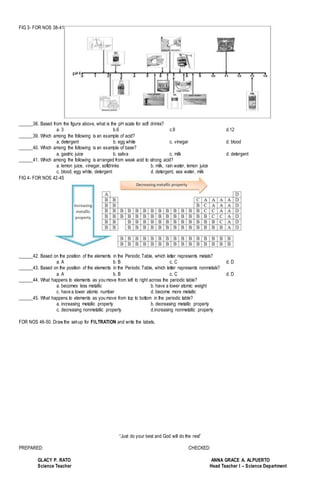
This document appears to be a science exam for 7th grade students covering topics in chemistry and the periodic table. It contains 50 multiple choice questions testing students' knowledge of mixtures, the periodic table, acids and bases, and other chemistry concepts. The questions are accompanied by figures to aid understanding. The exam also provides spaces for students to write their name, section, score, and date. It aims to assess students' understanding of fundamental chemistry principles through multiple choice questions.