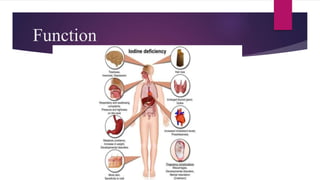
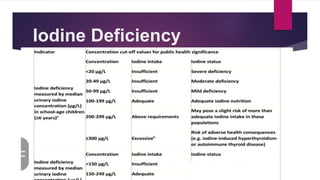
Iodine is an essential mineral needed to produce thyroid hormones. It regulates metabolism and is obtained through foods like seafood, dairy, and iodized salt. Without enough iodine, the thyroid gland can become underactive or overactive, leading to hypothyroidism or hyperthyroidism. Iodine is absorbed in the gastrointestinal tract and used by the thyroid gland to produce thyroid hormones, with excess excreted in urine.






![Iodine chemical form
atomicatomic weight number
acid-base properties symbolof higher-valence oxides53
[Kr]4d105s25p5 iodine
electroncrystal structure configuration physical state name at 20 oc (6B OF)
Halogens
Solid
Orthorhombic
Strongly acidic Brit
Phase at STP
solid
Melting point
(b) 386.85 K
(113.7 O C, 236.66 O F)
Boiling point
(12) 457.4 K (184.3 o c, 363.7 OF)
Density (near r.t.)
4.933 g/cm 3
Triple point
386.65 K, 12.1 kPa
Critical point
819 K, 11.7 MPa
Heat of fusion
(12) 15.52 kJ/mol
Heat of vaporisation
(12) 41.57 kJ/mol](https://image.slidesharecdn.com/1-231020201710-8f1c35a3/85/1-pptx-10-320.jpg)

