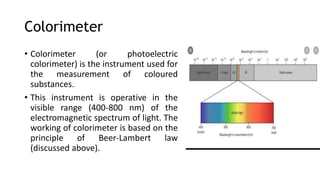
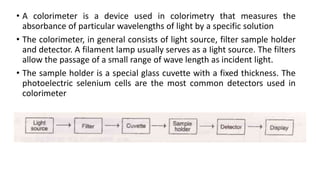
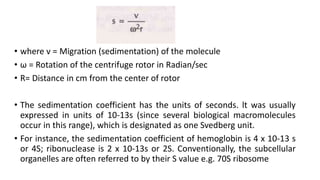
Photometry broadly deals with the study of light absorption by molecules in solution. It is used to quantitatively measure compounds in biological samples using the principle that monochromatic light passing through a solution will be absorbed at levels dependent on the concentration and thickness of absorbing materials, as described by Beer's and Lambert's laws. Instruments like colorimeters and spectrophotometers measure the absorbance of specific light wavelengths to determine concentrations, operating on these photometric principles.