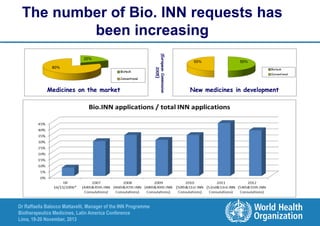
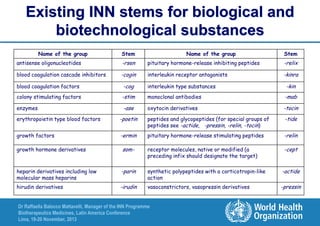
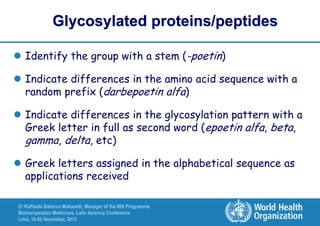
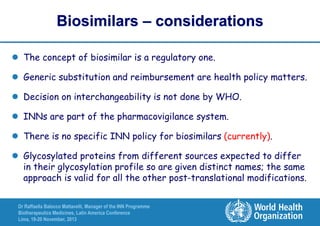
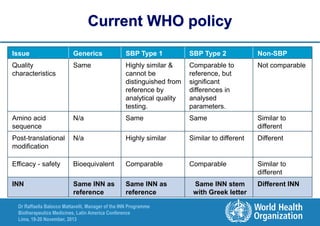
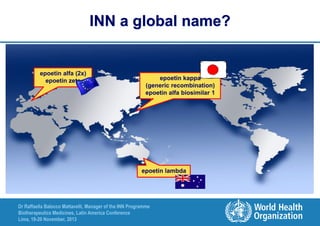
The document summarizes key points about the WHO International Nonproprietary Name (INN) Programme:
- The INN Programme assigns a single international nonproprietary name to pharmaceutical substances to ensure consistency worldwide and promote safe use of medicines globally.
- It aims to develop, establish, and promote international standards for naming pharmaceutical and biological products, including biotherapeutics.
- There is an ongoing discussion around developing a system for distinguishing biosimilar products through INN naming to promote unambiguous identification of reference products globally.