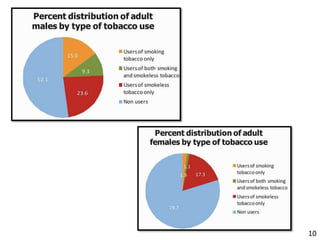
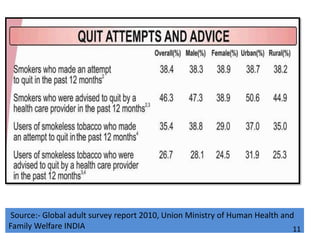
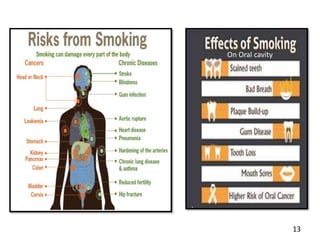
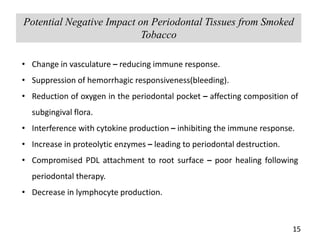
This document provides an overview of a tobacco cessation programme, including:
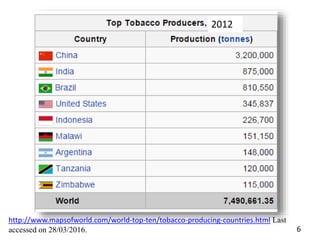
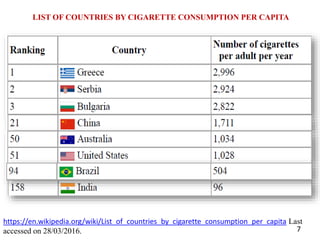
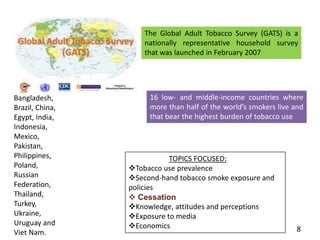
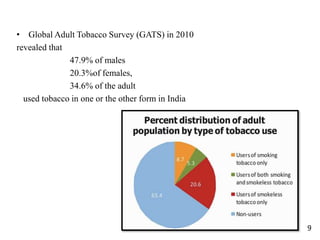
- Details on tobacco production, consumption, and the Global Adult Tobacco Survey.
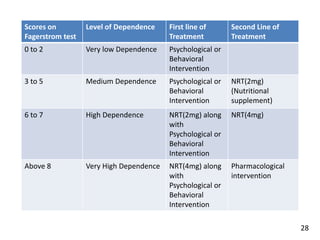
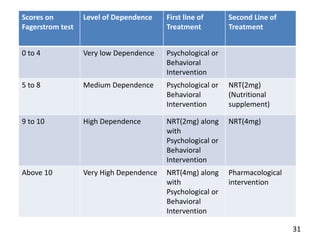
- Scales to measure nicotine dependence like the Fagerstrom test.
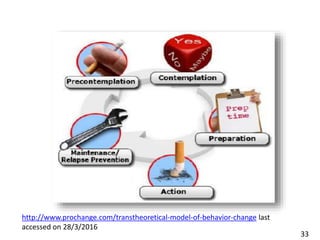
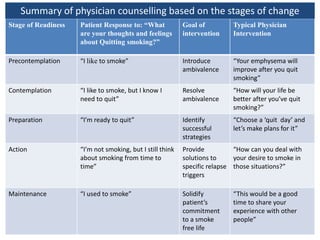
- Models of behavior change like the Transtheoretical Model.
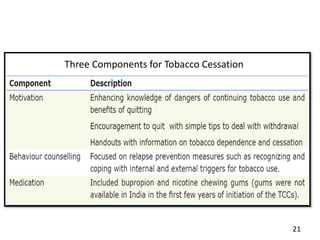
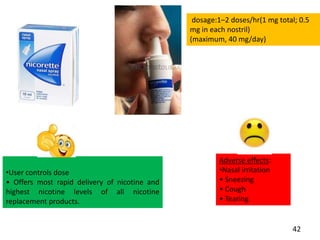
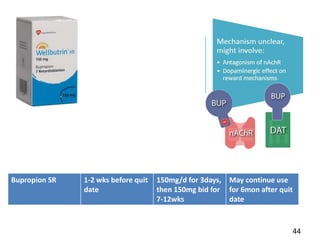
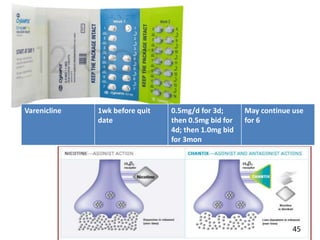
- Approaches to cessation like nicotine replacement therapy, pharmacotherapy, and behavioral counseling.
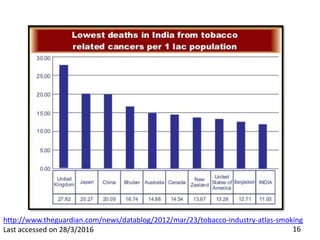
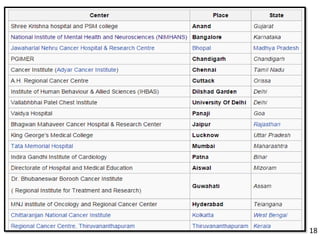
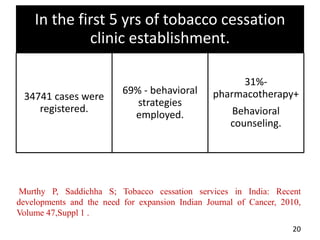
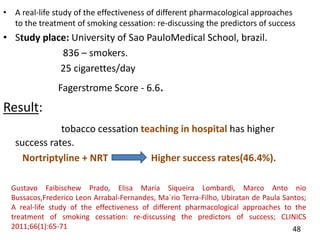
- Studies showing the success of tobacco cessation programs in India, including higher success rates for programs involving hospitals, counseling, and certain drug combinations.
- Barriers to cessation like a lack of trained health professionals and knowledge about tobacco's harms.