The document describes a new humidity sensor developed using graphene quantum dots (GQDs) synthesized from citric acid. The sensor exhibited high sensitivity to changes in relative humidity over a wide range from 0% to 97%. The sensor response decreased as humidity increased, unlike previous GQD sensors, suggesting a different sensing mechanism. The sensor response time was about 10 seconds while recovery time was longer, especially for larger humidity changes. The sensor demonstrated good repeatability, durability, and negligible hysteresis.
![Sensors and Actuators B 222 (2016) 728–734
Contents lists available at ScienceDirect
Sensors and Actuators B: Chemical
journal homepage: www.elsevier.com/locate/snb
A new humidity sensor based upon graphene quantum dots prepared
via carbonization of citric acid
Taher Alizadeha,∗
, Mahrokh Shokrib
a
Department of Analytical Chemistry, Faculty of Chemistry, University College of Science, University of Tehran, P.O. Box 14155-6455, Tehran, Iran
b
Department of Applied Chemistry, Faculty of Science, University of Mohaghegh Ardabili, Iran
a r t i c l e i n f o
Article history:
Received 15 May 2015
Received in revised form 5 August 2015
Accepted 29 August 2015
Available online 1 September 2015
Keywords:
Graphene quantum dot
Humidity
Sensor
Citric acid
a b s t r a c t
A new humidity sensor was fabricated using graphene quantum dots (GQDs), synthesized via controlled
carbonization of citric acid. The synthesized GQDs showed an outstandingly sensitivity to the varia-
tion of environment humidity. The sensor exhibited two sensing regions situating between 0%–52%
and 52%–97% of the relative humidity, indicating the dominance of two different sensing mechanisms.
However, increasing of the relative humidity from 0% to 97% led to about 1387-fold variation in sensor
response. Based on the results obtained, a sensing mechanism was proposed for the developed GQD-
based humidity sensor. The response time of the sensor was about 10 s. However, the recovery time was
found to be somewhat longer, compared to the response time. The sensor showed appropriate durability
and reversibility as well as negligible hysteresis, responding to the change in relative humidity of the
environment.
© 2015 Elsevier B.V. All rights reserved.
1. Introduction
Humidity is an important characteristic of the environment
which drastically influences all things in the environment. Fur-
thermore, it affects drastically many industries and technologies.
The determination and continuous monitoring of humidity in
numerous fields such as semiconductor manufacturing, packag-
ing industry, soil moisture monitoring, pharmaceutical and food
processing, civil engineering, electronic processing, household
appliances and air conditioning systems is necessary [1,2].
Electrical parameters of some materials such as ceramics, poly-
mers, metal oxides and their related composites can be varied as
a result of their exposing to humidity. These characteristics of the
described materials have been already utilized for the sensing of
humidity [3–8].
Carbon nanomaterials such as carbon nanotubes and graphene
have shown to be the ideal candidates in sensing field [9–13].
Graphene as a two dimensional carbon nanomaterial is an ideal
choice for sensing applications owing to large surface-to-volume
ratio and special electrical property [14,15].
∗ Corresponding author.
E-mail address: talizadeh@ut.ac.ir (T. Alizadeh).
Recently, a new generation of humidity sensors has been
introduced based on graphene, graphene oxide and reduced
graphene oxide. These kinds of humidity sensors have improved
the sensitivity and response time of the humidity sensors. Fur-
thermore they have led to production of flexible and miniaturized
sensors. Most of the reported cases are based on the change in the
electrical characteristic of graphene oxide [16] or reduced graphene
oxide [17–19] when exposing to humidified atmosphere. However,
graphene oxide thin film coated quartz crystal microbalance [20]
and optical graphene oxide sensor [21] have also been reported for
sensing of relative humidity.
Graphene quantum dot, prepared by conversion of graphene
nano-ribbons via oxidative edge-roughening and cleavage, has
recently been reported as an excellently sensitive humidity sen-
sor [22]. This sensor has exhibited an excellent sensitivity between
0% and 40% of the relative humidity. The mechanism has been pos-
tulated as the disturbance of electron tunneling between graphene
quantum dot layers as a result of water molecule adsorption and
the subsequent swelling of the GQD layers. An increase in the elec-
trical resistance of the sensor has been observed when exposing of
the sensor to humidity [22].
In this approach, we prepared graphene quantum dots via con-
trolled carbonization of citric acid [23]. We found that these kinds
of graphene quantum dots exhibited excellent sensitivity to rela-
tive humidity in wider relative humidity range, compared to the
http://dx.doi.org/10.1016/j.snb.2015.08.122
0925-4005/© 2015 Elsevier B.V. All rights reserved.](https://image.slidesharecdn.com/1-s2-180131204458/75/1-s2-0-s0925400515302999-main-1-2048.jpg)
![T. Alizadeh, M. Shokri / Sensors and Actuators B 222 (2016) 728–734 729
previously reported humidity sensor. The electrical resistance of
the citric acid derived GQD decreased as a result of increment in
relative humidity, conversely to the previous report. This suggested
a different sensing mechanism, which discussed in this approach.
2. Experimental
2.1. Materials
Citric acid monohydrate was purchased from (Merck, Germany).
Hydrazine hydrate (50–60%, reagent grade) was from Sigma Aldrich
Company (USA). Saturated solutions of sodium hydroxide (NaOH),
lithium chloride (LiCl), potassium acetate (CH3COOK), magnesium
chloride (MgCl2), potassium carbonate (K2CO3), magnesium nitrate
(Mg(NO3)2), sodium nitrite (NaNO2), sodium chloride (NaCl),
potassium chloride (KCl) and potassium sulfate (K2SO4), used for
adjusting the relative humidity of the sensing environment, were
prepared from their related solids of analytical grade, purchased
from (Merck, Germany).
2.2. Synthesis of GQDs
Graphene quantum dots were prepared by pyrolyzing of citric
acid according to the procedure reported by Chi et al. [23]. In a
typical procedure, 2 g of citric acid was put into a 5 mL beaker and
warmed to 200 ◦C. After 35 min, the color of the melted product
became orange, hinting the formation of GQDs [23]. The obtained
orange liquid was divided into three parts. The pHs of these parts
were adjusted to 5, 7.0 and 9.0, respectively, using NaOH solution
(0.1 M).
2.3. Sensor fabrication and its use for relative humidity sensing
In order to prepare the humidity sensor, little amount of
graphene quantum dot solution (0.1 mg mL−1) was dropped (4 L,
using a micropipette) on the interdigitated electrodes, deposited
on a silicon wafer (10 pairs of Pt electrodes having line widths of
0.5 mm and electrode to electrode spacing of about 0.3 mm). After
putting of GQD solution on the electrodes, it was transferred into
an oven, fixed at 50 ◦C and stored over there for 24 h.
A schematic diagram of the experimental setup, utilized for the
testing of the sensor in various relative humidity (RH) is shown in
Fig. 1. The humidity sensing property (the response of the sensor
as a function of time) was investigated by exposing the sensor to
various RH, achieved by saturated solutions of different salts and
phosphorus pentoxide (P2O5) powder. Saturated aqueous solutions
of NaOH, LiCl, CH3COOK, MgCl2, K2CO3, Mg(NO3)2, NaNO2, NaCl,
KCl and K2SO4 in a closed vessel were used to yield nearly 8%, 11%,
23%, 33%, 43%, 52%, 64%, 75%, 85% and 97% RH levels, respectively.
Dry environment (RH 0%) was established by P2O5 powder. The
sensing experiments were carried out at an ambient temperature
of 250. The electrical characteristic of the sensors (electrical resis-
tance of the sensors at applied potential of 1 V and frequency of
1 kHz) was measured by a digital multimeter, (E4980AL, Keysight
Technologies) connected to a PC at various relative humidity values.
3. Result and discussion
3.1. Characterization of graphene quantum dots
Schematic representation of the synthesis method of graphene
quantum dots by controlled carbonization of citric acid is illustrated
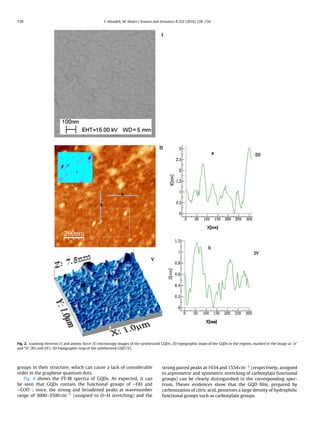
in Fig. 1. The scanning electron and atomic force microscopy images
of the synthesized GQDs are also shown in Fig. 2 (I) and (II), respec-
tively. As can be seen, the scanning electron microscopy image
Fig. 1. Schematic representation of the synthesis method of graphene quantum dots
by controlled carbonization of citric acid.
does not provide clear representation of the synthesized material
because of very small size of the synthesized GQDs. However, the
atomic force microscopy image of the GQDs (shown in Fig. 2(II))
and its related 2D (Fig. 2(III) and (IV)) and 3D (Fig. 2(V)) topographic
maps are clear enough to give useful information about the GQDs.
According to these illustrations, the average width of the GQDs was
calculated to be about 17 nm. Furthermore, the height of GQDs par-
ticles in the image was estimated to vary from 0.5 to 3 nm. These
results are in good agreement to the data reported by [23].
The XRD patterns of both graphite and GQDs are shown in Fig. 3.
It can be seen that the positions of main peaks (0 0 2) of GQDS
and graphite in axis direction (2 theta) are the same; although, the
peak, related to graphite, is sharper than that of GQDs. This clearly
suggests that the carbonization of citric acid leads to graphite like
structures. However, the absence of sharp peak in the case of XRD
pattern of the GQDs can be related to the disordered structure of
GQDs, as a result of presence of great deal of oxygenated functional](https://image.slidesharecdn.com/1-s2-180131204458/85/1-s2-0-s0925400515302999-main-2-320.jpg)
![T. Alizadeh, M. Shokri / Sensors and Actuators B 222 (2016) 728–734 733
Fig. 9. The temperature dependent response of the GQDs based sensor in two relative humidity ranges of 0–52% (I) and 52–97% (II).
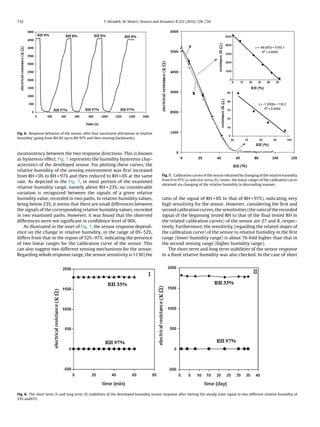
term stability test, the sensor was exposed to a fixed relative humid-
ity and the obtained steady state response (after about 20 s) was
recorded between times intervals of 5 min. The checking of long
term stability of the sensor was similar to that of short term stabil-
ity, except that in this case, the sensor response to the given relative
humidity was examined every few days. The results obtained are
shown in Fig. 8. It can be seen that no significant variation in the
sensor response is observed upon about 1 h exposing of the sensor
to relative humidity of 33% and 97%. Furthermore, the long term sta-
bility of the sensor is satisfactory; since, after more than 1 month,
the response of the sensor to the tested relative humidity levels is
unchanged.
Fig. 9(I) and (II) shows the temperature dependent response of
the GQDs-based sensor in two relative humidity ranges of 0%–52%
and 52%–97%, respectively. As the temperature increases, the RH
dependent curves (in both humidity ranges) shift to lower elec-
trical resistance. The mean temperature coefficient at the tested
temperature range of 15–35 ◦C was calculated to be −0.52% and
−0.43% RH/◦C over the humidity range of 0%–52% RH and 52%–97%,
respectively.
3.4. Humidity response mechanism
As described in Fig. 7, the GQDs based sensing film represents
two humidity sensing ranges. This suggests two different mech-
anisms dominating the sensor response to the relative humidity.
The firs range is between 0% and 52% and the second is from 52% to
97%. In the first described range, the sensor is 76-fold sensitive than
that in the second range. Graphene quantum dots can be consid-
ered as p-type semiconductors [11,24]. However, according to the
FT-IR spectrum, the prepared GQDs include so many carboxylate
functional groups. The adsorption of water molecules to graphene
quantum dots may be rather facilitated via negatively charged car-
boxylate groups than the OH functionalities of GQDs. Adsorption of
water molecules, acting as electron withdrawing compound [19],
decrease the density of electron on the GQDs. This, subsequently,
leads to an increase in the number of the hole-type carriers in
p-type semiconductor of GQD, decreasing thus the electrical resis-
tance of the sensing film of the GQDs.
However, in the higher values of the relative humidity, other
sensing mechanism (described in the following) may be dominant.
At high RH values, the multilayer water molecules, adsorbed on
the GQDs, may be ionized to produce hydronium ions (H3O+) as
the charge carriers. The protons transport via ionic conductivity
can cause a decrease in sensor resistance [19].
4. Conclusion
In summary, a new humidity sensor based on the graphene
quantum dots, synthesized via carbonization of citric acid, was
introduced. The sensor was highly sensitive to change in rela-
tive humidity in wide range. Other than its higher sensitivity, the
capability of this sensor to be active in lower relative humidity val-
ues can be cited as an advantage of this sensor over most of the
previously reported humidity sensors. The sensor exhibited short
response time and no considerable hysteresis was observed for the
sensor. Since two distinct sensing ranges were seen for this sen-
sor, we supposed two sensing mechanism, dominating the sensor
response to relative humidity.
References
[1] A. Wexler, Humidity and Moisture Measurement and control in Science and
Industry, Reinhold, New York, 1965, pp. 219–414.
[2] B. Patissier, Humidity sensors for automotive, appliances and consumer
applications, Sens. Actuators B 59 (1999) 231–234.
[3] B.M. Kulwicki, Humidity sensors, J. Am. Ceram. Soc. 74 (1991) 697–708.
[4] Z. Chen, C. Lu, Humidity sensors: view of materials and mechanism, Sens. Lett.
3 (2005) 274–295.
[5] N. Yamazoe, Y. Shimizu, Humidity sensors: principles and applications, Sens.
Actuators 10 (1986) 379–398.
[6] E. Traversa, Ceramic sensors for humidity detection: the state-of-the-art and
future developments, Sens. Actuators B 23 (1995) 135–156.
[7] Y.C. Yeh, T.Y. Tseng, D.A. Chang, Electrical properties of TiO2–K2Ti6O13 porous
ceramic humidity sensor, J. Am. Ceram. Soc. 73 (1990) 1992–1998.
[8] H. Grange, C. Bieth, H. Boucher, G. Delapierre, A capacitive humidity sensor
with very fast response time and very low hysteresis, Sens. Actuators 12
(1987) 291–296.
[9] T. Alizadeh, F. Rezaloo, A new chemiresistor sensor based on a blend of carbon
nanotube, nano-sized molecularly imprinted polymer and poly methyl
methacrylate for the selective and sensitive determination of ethanol vapor,
Sens. Actuators B 176 (2013) 28–37.
[10] T. Alizadeh, S. Mirzagholipur, A Nafion-free non-enzymatic amperometric
glucose sensor based on copper oxide nanoparticles–graphene
nanocomposite, Sens. Actuators B 198 (2014) 438–447.
[11] J.T. Robinson, F.K. Perkins, E.S. Snow, Z. Wei, P.E. Sheehan, Reduced graphene
oxide molecular sensors, Nano Lett. 8 (2008) 3137–3140.
[12] R.K. Paul, S. Badhulika, N.M. Saucedo, A. Mulchandani, Graphene nanomesh as
highly sensitive chemiresistor gas sensor, Anal. Chem. 84 (2012) 8171–8178.
[13] J.W. Han, B. Kim, J. Li, M. Meyyappan, Carbon nanotube based humidity sensor
on cellulose paper, J. Phys. Chem. C 116 (2012) 22094–22097.
[14] T. Alizadeh, L.H. Soltani, Graphene/poly(methyl methacrylate) chemiresistor
sensor for formaldehyde odor sensing, J. Hazard. Mater. 248–249 (2013)
401–406.](https://image.slidesharecdn.com/1-s2-180131204458/85/1-s2-0-s0925400515302999-main-6-320.jpg)
![734 T. Alizadeh, M. Shokri / Sensors and Actuators B 222 (2016) 728–734
[15] S. Basua, P. Bhattacharyya, Recent developments on graphene and graphene
oxide based solid state gas sensors, Sens. Actuators B 173 (2012) 1–21.
[16] H.W. Yu, H.K. Kim, T. Kim, K.M. Bae, S.M. Seo, J.M. Kim, T.J. Kang, Y.H. Kim,
Self-powered humidity sensor based on graphene oxide composite film
intercalated by poly(sodium 4-styrenesulfonate), ACS Appl. Mater. Interfaces
6 (2014) 8320–8326.
[17] D. Zhang, J. Tong, B. Xia, Humidity-sensing properties of chemically reduced
grapheneoxide/polymer nanocomposite film sensor based on layer-by-layer
nano self-assembly, Sens. Actuators B 197 (2014) 66–72.
[18] T. Fu, J. Zhu, M. Zhuo, B. Guan, J. Li, Z. Xu, Q. Li, Humidity sensors based on
graphene/SnOx/CF nanocomposites, J. Mater. Chem. C 2 (2014) 4861–4866.
[19] P.G. Su, C.F. Chiou, Electrical and humidity-sensing properties of reduced
graphene oxide thin film fabricated by layer-by-layer with covalent
anchoring on flexible substrate, Sens. Actuators B 200 (2014) 9–18.
[20] Y. Yao, X. Chen, H. Guo, Z. Wu, Graphene oxide thin film coated quartz crystal
microbalance for humidity detection, Appl. Surf. Sci. 257 (2011) 7778–7782.
[21] W.H. Lim, Y.K. Yap, W.Y. Chong, H. Ahmad, All-optical graphene oxide
humidity sensors, Sensors 14 (2014) 24329–24337.
[22] T.S. Sreeprasad, A.A. Rodriguez, J. Colston, A. Graham, E. Shishkin, V. Pallem, V.
Berry, Electron-tunneling modulation in percolating network of graphene
quantum dots: fabrication, henomenological understanding, and
humidity/pressure sensing applications, Nano Lett. 13 (2013) 1757–1763.
[23] Y. Dong, J. Shao, C. Chen, H. Li, R. Wang, Y. Chi, X. Lin, G. Chen, Blue
luminescent graphene quantum dots and graphene oxide prepared by tuning
the carbonization degree of citric acid, Carbon 50 (2012) 4738–4743.
[24] C.O. Kim, S.W. Hwang, S. Kim, D.H. Shin, S.S. Kang, J.M. Kim, C.W. Jang, J.H.
Kim, K.W. Lee, S.H. Choi, E. Hwang, High-performance graphene quantum dot
photodetectors, Sci. Rep. 4 (2014) 5603.
Biographies
Taher Alizadeh received the BS degree from the Univer-
sity of Guilan, Rasht, Iran, in 1999. He received MS degree
from the University of Tabriz, Iran, in 2002 and Ph.D.
degree from the University of Tehran, Iran, in 2006. He was
with University of Mohaghegh Ardabili, from September
2006 to October 2013. Now, he is continuing his research
activities as an associated professor in Department of Ana-
lytical Chemistry, Faculty of Chemistry, University Collage
of Science, University of Tehran, Iran. His current research
interests include the development of chemical sensors and
electronic nose based on new materials, Electrochemistry
and molecular/Ionic imprinting technology.
Mahrokh Shokri received the BS degree from the Uni-
versity of Mohaghegh Ardabili in 2011. She received
MS degree in analytical chemistry from Department of
Applied Chemistry, University of Mohaghegh Ardabili,
Ardabil, Iran, in 2014.](https://image.slidesharecdn.com/1-s2-180131204458/85/1-s2-0-s0925400515302999-main-7-320.jpg)